Abstract
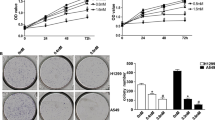
An increasing amount of evidence has demonstrated the anticancer activity of triterpenes extracted from traditional medicines. Echinocystic acid (EA), a natural triterpene isolated from Eclipta prostrata (L.) L., has previously been shown to exhibit anticancer activity in HepG2 and HL-60 cells. The aim of the present study was to investigate the anticancer activity of EA in non-small cell lung cancer (NSCLC) cells. For this purpose, the viability and proliferation of A549 cells were determined using a Cell Counting Kit-8 and 5-ethynyl-2′-deoxyuridine staining. The migratory and invasive ability of the A549 cells were measured using wound healing and Transwell assays. Hoechst staining was also performed to detect the apoptosis of A549 cells. The proliferation of A549 cells and the distributions of different growth phases were determined using a flow cytometer. Western blot analysis was used to detect the expression levels of cyclin D, partitioning defective 3 homolog (Par3), PI3K, Akt, mTOR, Bax, Bcl-2 and caspase-3. EA inhibited the proliferation, and the migratory and invasive abilities of cultured lung carcinoma cells (A549 cells), and induced cell cycle arrest in the G1 phase of the cell cycle. Treatment with EA upregulated Par3 expression and inhibited the PI3K/Akt/mTOR pathway in vitro. In addition, EA treatment inhibited tumor growth, suppressed proliferation and induced the apoptosis of tumor cells in NSCLC tumor xenografts in mice. On the whole, these results suggest that EA may represent a potential therapeutic agent for NSCLC.







Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- DMEM:
-
Dulbecco’s Modified Eagle’s Medium
- EA:
-
Echinocystic acid
- NSCLC:
-
Non-small cell lung cancer cells
- OD:
-
Optical density
- p-Akt:
-
Phosphorylated Akt
- SCLC:
-
Small cell lung cancer
- Par3:
-
Partitioning defective 3 homolog
- TBST:
-
Tris buffer with 0.05% (v/v) Tween-20
References
Gallant JN, Lovly CM. Established, emerging and elusive molecular targets in the treatment of lung cancer. J Pathol. 2018;244:565–77.
Fiordoliva I, Meletani T, Baleani MG, Rinaldi S, Savini A, Di Pietro PM, Berardi R. Managing hyponatremia in lung cancer: latest evidence and clinical implications. Ther Adv Med Oncol. 2017;9:711–9.
Jung CY, Antonia SJ. Tumor immunology and immune checkpoint inhibitors in non-small cell lung cancer. Tuberc Respir Dis (Seoul). 2018;81:29–41.
Mathew M, Enzler T, Shu CA, Rizvi NA. Combining chemotherapy with PD-1 blockade in NSCLC. Pharmacol Ther. 2018;186:130–7.
Tseng CY, Lin CH, Wu LY, Wang JS, Chung MC, Chang JF, Chao MW. Potential combinational anti-cancer therapy in non-small cell lung cancer with traditional Chinese medicine Sun-Bai-Pi extract and cisplatin. PLoS ONE. 2016;11:e0155469.
Chudzik M, Korzonek-Szlacheta I, Krol W. Triterpenes as potentially cytotoxic compounds. Molecules. 2015;20:1610–25.
Tong X, Lin S, Fujii M, Hou DX. Molecular mechanisms of echinocystic acid-induced apoptosis in HepG2 cells. Biochem Biophys Res Commun. 2004;321:539–46.
Tong X, Lin S, Fujii M, Hou DX. Echinocystic acid induces apoptosis in HL-60 cells through mitochondria-mediated death pathway. Cancer Lett. 2004;212:21–32.
Lee JS, Ahn JH, Cho YJ, Kim HY, Yang YI, Lee KT, Jang DS, Choi JH. α-Terthienylmethanol, isolated from Eclipta Prostrata, induces apoptosis by generating reactive oxygen species via NADPH oxidase in human endometrial cancer cells. J Ethnopharmacol. 2015;169:426–34.
Kim HY, Kim HM, Ryu B, Lee JS, Choi JH, Jang DS. Constituents of the aerial parts of Eclipta Prostrata and their cytotoxicity on human ovarian cancer cells in vitro. Arch Pharm Res. 2015;38(11):1963–9.
Wang T, Si XQ, Zhou GL, Dai R, Zhou G, Cao D, Yang C. In vivo anti-tumor effect and in vitro anti-angiogenic effect of alcohol extract from Euphorbia prostrata. Zhongguo Zhong Yao Za Zhi. 2017;42(9):1722–9.
Shumin D, Xuefeng H, Fujing W, Gang W, Xiaobin T, Ying L, Yuanli Z, Huihui Q, Sun E, Nan J, Zihao L, Jie S, Liang F, Xiaobin J. Regulation of Eclipta prostrata L. components on cigarette smoking-induced autophagy of bronchial epithelial cells via keap1-Nrf2 pathway. Environ Toxico. 2018;2018:4.
Joh EH, Gu W, Kim DH. Echinocystic acid ameliorates lung inflammation in mice and alveolar macrophages by inhibiting the binding of LPS to TLR4 in NF-kappaB and MAPK pathways. Biochem Pharmacol. 2012;84:331–40.
Zhou J, Zhou W, Kong F, Xiao X, Kuang H, Zhu Y. microRNA-34a overexpression inhibits cell migration and invasion via regulating SIRT1 in hepatocellular carcinoma. Oncol Lett. 2017;14:6950–4.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCq method. Methods. 2001;25:402–8.
Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DAH, Glennie MJ, Kelland LR, Robinson V, Stratford IJ, Tozer GM, Watson S, Wedge SR, Eccles SA. An ad hoc committee of the National Cancer Research Institute. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010;102:1555–77.
Tong S, Xia T, Fan K, Jiang K, Zhai W, Li JS, Wang SH, Wang JJ. Loss of Par3 promotes lung adenocarcinoma metastasis through 14–3–3ζ protein. Oncotarget. 2016;7(39):64260–73.
Wang L, Kong W, Liu B, Zhang X. Proliferating cell nuclear antigen promotes cell proliferation and tumorigenesis by up-regulating STAT3 in non-small cell lung cancer. Biomed Pharmacother. 2018;104:595–602.
Ogunbinu AO, Flamini G, Cioni PL, Ogunwande IA, Okeniyi SO. Essential oil constituents of Eclipta prostrata (L.) L. and vernonia amygdalina delile. Nat Prod Commun. 2009;4:421–4.
Lee MK, Ha NR, Yang H, Sung SH, Kim GH, Kim YC. Antiproliferative activity of triterpenoids from Eclipta prostrata on hepatic stellate cells. Phytomedicine. 2008;15:775–80.
Dhandapani R. Hypolipidemic activity of Eclipta prostrata (L.) L. leaf extract in atherogenic diet induced hyperlipidemic rats. Indian J Exp Biol. 2007;45:617–9.
Liu X, Jiang Y, Zhao Y, Tang H. Effect of ethyl acetate extract of Eclipta prostrata on mice of normal and immunosupression. Zhong Yao Cai. 2000;23:407–9.
Sawant M, Isaac JC, Narayanan S. Analgesic studies on total alkaloids and alcohol extracts of Eclipta alba (Linn) Hassk. Phytother Res. 2004;18:111–3.
Mors WB, do Nascimento MC, Parente JP, da Silva MH, Melo PA, Suarez-Kurtz G. Neutralization of lethal and myotoxic activities of South American rattlesnake venom by extracts and constituents of the plant Eclipta prostrata (Asteraceae). Toxicon. 1989;27:1003–9.
Kim DI, Lee SH, Choi JH, Lillehoj HS, Yu MH, Lee GS. The butanol fraction of Eclipta prostrata (Linn) effectively reduces serum lipid levels and improves antioxidant activities in CD rats. Nutr Res. 2008;28:550–4.
Tewtrakul S, Subhadhirasakul S, Tansakul P, Cheenpracha S, Karalai C. Antiinflammatory constituents from Eclipta prostrata using RAW264.7 macrophage cells. Phytother Res. 2011;25:1313–6.
Xia X, Yu R, Wang X, Wei M, Li Y, Wang A, Ma Y, Zhang J, Ji Z, Li Y, Wang Q. Role of Eclipta prostrata extract in improving spatial learning and memory deficits in D-galactose-induced aging in rats. J Tradit Chin Med. 2019;39(5):649–57.
Zhang JS, Guo QM. Studies on the chemical constituents of Eclipta prostrata (L). Yao Xue Xue Bao. 2001;36:34–7.
Cho YJ, Woo JH, Lee JS, Jang DS, Lee KT, Choi JH. Eclalbasaponin II induces autophagic and apoptotic cell death in human ovarian cancer cells. J Pharmacol Sci. 2016;132:6–14.
Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT, International Natural Product Sciences Taskforce. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20(3):200–16.
Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–22.
McCaffrey LM, Montalbano J, Mihai C, Macara IG. Loss of the Par3 polarity protein promotes breast tumorigenesis and metastasis. Cancer Cell. 2016;30:351–2.
Song T, Tian X, Kai F, Ke J, Wei Z, Jing-Song L, Si-Hua W, Jian-Jun W. Loss of Par3 promotes lung adenocarcinoma metastasis through 14-3-3zeta protein. Oncotarget. 2016;7:64260–73.
Seminario-Vidal L, Kreda S, Jones L, O’Neal W, Trejo J, Boucher RC, Lazarowski ER. Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of rho- and Ca2+-dependent signaling pathways. J Biol Chem. 2009;284:20638–48.
Tan AC. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC). Thorac Cancer. 2020;11(3):511–8.
Tewari D, Patni P, Bishayee A, Sah AN, Bishayee A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: a novel therapeutic strategy. Semin Cancer Biol. 2019;2019:30405–15.
McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Montalto G, Cervello M, Nicoletti F, Fagone P, Malaponte G, Mazzarino MC, Candido S, Libra M, Basecke J, Mijatovic S, Maksimovic-Ivanic D, Milella M, Tafuri A, Cocco L, Evangelisti C, Chiarini F, Martelli AM. Mutations and deregulation of Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascades which alter therapy response. Oncotarget. 2012;3:954–87.
Krencz I, Sebestyen A, Khoor A. mTOR in lung neoplasms. Pathol Oncol Res. 2020;26(1):35–48.
Cheng H, Shcherba M, Pendurti G, Liang Y, Piperdi B, Perez-Soler R. Targeting the PI3K/AKT/mTOR pathway: potential for lung cancer treatment. Lung Cancer Manag. 2014;3:67–75.
Song M, Bode AM, Dong Z, Lee MH. AKT as a therapeutic target for cancer. Cancer Res. 2019;79(6):1019–31.
Mohan CD, Srinivasa V, Rangappa S, Mervin L, Mohan S, Paricharak S, Baday S, Li F, Shanmugam MK, Chinnathambi A, Zayed ME, Alharbi SA, Bender A, Sethi G, Rangappa KS. Trisubstituted-Imidazoles induce apoptosis in human breast cancer cells by targeting the oncogenic PI3K/Akt/mTOR signaling pathway. PLoS ONE. 2016;11(4):e0153155.
Xiao S, Wang Qi, Si L, Shi Y, Wang H, Fei Yu, Zhang Y, Li Y, Zheng Y, Zhang C, Wang C, Zhang L, Zhou D. Synthesis and anti-HCV entry activity studies of β-cyclodextrin-pentacyclic triterpene conjugates. ChemMedChem. 2014;9(5):1060–70.
Zhao Z, Matsunami K, Otsuka H, Shinzato T, Takeda Y, Kawahata M, Yamaguchi K, Schefflerins AG. New triterpene glucosides from the leaves of Schefflera arboricola. Chem Pharm Bull (Tokyo). 2010;58(10):1343–8.
Keller M, Fankhauser S, Giezendanner N, König M, Keresztes F, Danton O, Fertig O, Marcourt L, Hamburger M, Butterweck V, Potterat O. Saponins from saffron corms inhibit the gene expression and secretion of pro-inflammatory cytokines. J Nat Prod. 2021;84(3):630–45.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Gansu Provincial Technological Science Foundation of China (Grant no. 1606RJZA040) and the Longyuan Youth Innovation and Entrepreneurship Talents (Team) Project (Grant no. PF2128001).
Author information
Authors and Affiliations
Contributions
DZ designed and performed experiments, and analyzed, interpreted and presented the results for group discussions. BL, PJ and CW were involved in the study methodology, in the description and analysis of the results, and prepared the figures for the manuscript. DZ, BL, PJ, FT and CW provided the rationale, background and framework of the study, and also provided feedback regarding the study. DZ and YL confirm the authenticity of all the raw data. DZ, BL, PJ, CW, FT and YL have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no competing interests.
Ethical approval
All animal protocols were approved by the Medical Animal Ethics Committee of Lanzhou University Second Hospital (Gan X2020J011), and were all performed according to the Federation of Laboratory Animal Science Associations (FELASA) guidelines for the definition of humane endpoints and the Arrive guidelines for animal care and protection (13).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12032_2023_2029_MOESM1_ESM.tif
Supplementary file1 (TIF 151 KB)—EA exhibits no effect on the cell viability of normal lung epithelial cell line (BEAS-2B).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, D., Li, B., Wang, C. et al. Echinocystic acid induces the apoptosis, and inhibits the migration and invasion of non-small cell lung cancer cells. Med Oncol 40, 182 (2023). https://doi.org/10.1007/s12032-023-02029-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02029-2