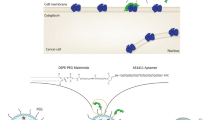
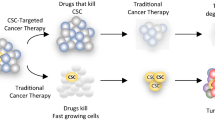
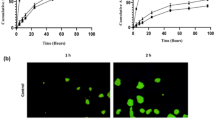
Abstract
Reprogrammed metabolism and active stemness contribute to cancer stem cells’ (CSCs) survival and tumorigenesis. LXR signaling regulates the metabolism of different cancers. A selective LXR inhibitor, SR9243 (SR), can target and eradicate non-CSC tumor cells. CD133 is a stem marker in solid tumors-associated CSCs within the active lipogenesis, and anti-CD133 mAb targeting liposomal drug delivery systems expected to increase drug internalization and improve the therapeutic efficacy of poor-in water solubility drugs, e, g., SR. In this study, anti-CD133 mAbs-targeted Immunoliposomes (ILipo) were developed for specific delivery of SR into MACS-enriched CD133 + CSCs and induce their functional effects. Results have shown that ILipo having an average size of 64.79 nm can encapsulate SR in maximum proportion, and cell association studies have shown cationic ILipo and targeting CD133 provide the CSCs binding. Also, FCM analysis of RhoB has demonstrated that the ILipo uptake was higher in CD133 + CSCs than in the non-targeted liposomes. ILipo-SR was significantly more toxic in CD133 + CSCs compared to the free SR and non-targeted ones. More efficient than Lipo-SR, ILipo-SR improved the reduction of clonogenicity, stemness, and lipogenesis in CD133 + CSCs in vitro, boosted ROS generation, and induced apoptosis. Our study revealed the dual targeting of CD133 and LXR appears to be a promising strategy for targeting CD133 + CSCs-presenting dynamic metabolism and self-renewal potentials.
Graphical abstract








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Abbreviations
- CSC:
-
Cancer stem cell
- CLs:
-
Cationic liposomes
- DOPE:
-
Dioleoyl phosphatidylethanolamine
- DOTAP:
-
1,2-Dioleoyl-3-trimethylammonium-propane
- FASN:
-
Fatty acid synthase
- ILipo:
-
Immunoliposomes
- LXR:
-
Liver X nuclear receptor
- MACS:
-
Magnetic activated cell sorting
- SCD1:
-
Stearoyl-CoA desaturase-1
References
Ehmsen S, Pedersen MH, Wang G, Terp MG, Arslanagic A, Hood BL, et al. Increased cholesterol biosynthesis is a key characteristic of breast cancer stem cells influencing patient outcome. Cell Rep. 2019;27(13):3927-38.e6.
Li H, Feng Z, He M-L. Lipid metabolism alteration contributes to and maintains the properties of cancer stem cells. Theranostics. 2020;10(16):7053.
Li J, Condello S, Thomes-Pepin J, Ma X, Xia Y, Hurley TD, et al. Lipid desaturation is a metabolic marker and therapeutic target of ovarian cancer stem cells. Cell Stem Cell. 2017;20(3):303-14.e5.
Fu T, Coulter S, Yoshihara E, Oh TG, Fang S, Cayabyab F, et al. FXR regulates intestinal cancer stem cell proliferation. Cell. 2019;176(5):1098–112.
Dianat-Moghadam H, Khalili M, Keshavarz M, Azizi M, Hamishehkar H, Rahbarghazi R, et al. Modulation of LXR signaling altered the dynamic activity of human colon adenocarcinoma cancer stem cells in vitro. Cancer Cell Int. 2021;21(1):100.
Jagust P, de Luxán-Delgado B, Parejo-Alonso B, Sancho P. Metabolism-based therapeutic strategies targeting cancer stem cells. Front Pharmacol. 2019;10:203.
Ferrand A, Sandrin MS, Shulkes A, Baldwin GS. Expression of gastrin precursors by CD133-positive colorectal cancer cells is crucial for tumour growth. Biochimica et Biophys Acta (BBA)—Mol Cell Res. 2009;1793(3):477–88.
Wang BB, Li ZJ, Zhang FF, Hou HT, Yu JK, Li F. Clinical significance of stem cell marker CD133 expression in colorectal cancer. Histol Histopathol. 2016;31(3):299–306.
Rappa G, Fargeas CA, Le TT, Corbeil D, Lorico A. Letter to the editor: an intriguing relationship between lipid droplets, cholesterol-binding protein CD133 and Wnt/β-catenin signaling pathway in carcinogenesis. Stem Cells. 2015;33(4):1366–70.
Dianat-Moghadam H, Heidarifard M, Jahanban-Esfahlan R, Panahi Y, Hamishehkar H, Pouremamali F, et al. Cancer stem cells-emanated therapy resistance: implications for liposomal drug delivery systems. J Control Release. 2018;288:62–83.
Shen Y-A, Li W-H, Chen P-H, He C-L, Chang Y-H, Chuang C-M. Intraperitoneal delivery of a novel liposome-encapsulated paclitaxel redirects metabolic reprogramming and effectively inhibits cancer stem cells in Taxol®-resistant ovarian cancer. Am J Trans Res. 2015;7(5):841.
Biju V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem Soc Rev. 2014;43(3):744–64.
Arabi L, Badiee A, Mosaffa F, Jaafari MR. Targeting CD44 expressing cancer cells with anti-CD44 monoclonal antibody improves cellular uptake and antitumor efficacy of liposomal doxorubicin. J Control Release. 2015;220:275–86.
Alshaer W, Hillaireau H, Vergnaud J, Ismail S, Fattal E. Functionalizing liposomes with anti-CD44 aptamer for selective targeting of cancer cells. Bioconjug Chem. 2014;26(7):1307–13.
Ning S-T, Lee S-Y, Wei M-F, Peng C-L, Lin SY-F, Tsai M-H, et al. Targeting colorectal cancer stem-like cells with anti-CD133 antibody-conjugated SN-38 nanoparticles. ACS Appl Mater Interfaces. 2016;8(28):17793–804.
Ganesh S, Iyer AK, Morrissey DV, Amiji MM. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials. 2013;34(13):3489–502.
Carpenter KJ, Valfort A-C, Steinauer N, Chatterjee A, Abuirqeba S, Majidi S, et al. LXR-inverse agonism stimulates immune-mediated tumor destruction by enhancing CD8 T-cell activity in triple negative breast cancer. Sci Rep. 2019;9(1):1–18.
Chen Z, Moon JJ, Cheng W. Quantitation and stability of protein conjugation on liposomes for controlled density of surface epitopes. Bioconjug Chem. 2018;29(4):1251–60.
Yang D, Correia JJ, Stafford WF III, Roberts CJ, Singh S, Hayes D, et al. Weak IgG self-and hetero-association characterized by fluorescence analytical ultracentrifugation. Protein Sci. 2018;27(7):1334–48.
Schneider M, Huber J, Hadaschik B, Siegers GM, Fiebig H-H, Schüler J. Characterization of colon cancer cells: a functional approach characterizing CD133 as a potential stem cell marker. BMC Cancer. 2012;12(1):96.
Dianat-Moghadam H, Sharifi M, Salehi R, Keshavarz M, Shahgolzari M, Amoozgar Z. Engaging stemness improves cancer immunotherapy. Cancer Lett. 2022;554:216007.
Dianat-Moghadam H, Mahari A, Salahlou R, Khalili M, Azizi M, Sadeghzadeh H. Immune evader cancer stem cells direct the perspective approaches to cancer immunotherapy. Stem Cell Res Ther. 2022;13(1):1–12.
Dianat-Moghadam H, Heidarifard M, Mahari A, Shahgolzari M, Keshavarz M, Nouri M, et al. TRAIL in oncology: From recombinant TRAIL to nano- and self-targeted TRAIL-based therapies. Pharmacol Res. 2020;155:104716.
Obeid MA, Tate RJ, Mullen AB, Ferro VA. Lipid-based nanoparticles for cancer treatment Lipid Nanocarriers for Drug Targeting. Netherlands: Elsevier; 2018. p. 313–59.
Flaveny CA, Griffett K, El-Gendy BE-DM, Kazantzis M, Sengupta M, Amelio AL, et al. Broad anti-tumor activity of a small molecule that selectively targets the Warburg effect and lipogenesis. Cancer Cell. 2015;28(1):42–56.
Uno S, Endo K, Jeong Y, Kawana K, Miyachi H, Hashimoto Y, et al. Suppression of β-catenin signaling by liver X receptor ligands. Biochem Pharmacol. 2009;77(2):186–95.
Agarwal JR, Wang Q, Tanno T, Rasheed Z, Merchant A, Ghosh N, et al. Activation of liver X receptors inhibits hedgehog signaling, clonogenic growth, and self-renewal in multiple myeloma. Mol Cancer Ther. 2014;13(7):1873–81.
Soema PC, Willems G-J, Jiskoot W, Amorij J-P, Kersten GF. Predicting the influence of liposomal lipid composition on liposome size, zeta potential and liposome-induced dendritic cell maturation using a design of experiments approach. Eur J Pharm Biopharm. 2015;94:427–35.
Laouini A, Jaafar-Maalej C, Limayem-Blouza I, Sfar S, Charcosset C, Fessi H. Preparation, characterization and applications of liposomes: state of the art. J Colloid Sci Biotechnol. 2012;1(2):147–68.
Majzoub RN, Chan C-L, Ewert KK, Silva BFB, Liang KS, Jacovetty EL, et al. Uptake and transfection efficiency of PEGylated cationic liposome–DNA complexes with and without RGD-tagging. Biomaterials. 2014;35(18):4996–5005.
Mahmoudi R, Dianat-Moghadam H, Poorebrahim M, Siapoush S, Poortahmasebi V, Salahlou R, et al. Recombinant immunotoxins development for HER2-based targeted cancer therapies. Cancer Cell Int. 2021;21:1–17.
Wattiaux R, Jadot M, Warnier-Pirotte M-T, Wattiaux-De CS. Cationic lipids destabilize lysosomal membrane in vitro. FEBS Lett. 1997;417(2):199–202.
Yasumoto Y, Miyazaki H, Vaidyan LK, Kagawa Y, Ebrahimi M, Yamamoto Y, et al. Inhibition of fatty acid synthase decreases expression of stemness markers in glioma stem cells. PLoS ONE. 2016;11(1):e0147717.
Semenza GL. Hypoxia-inducible factors: coupling glucose metabolism and redox regulation with induction of the breast cancer stem cell phenotype. EMBO J. 2017;36(3):252–9.
Noto A, Raffa S, De Vitis C, Roscilli G, Malpicci D, Coluccia P, et al. Stearoyl-CoA desaturase-1 is a key factor for lung cancer-initiating cells. Cell Death Dis. 2013;4(12):e947-e.
Tirinato L, Liberale C, Di Franco S, Candeloro P, Benfante A, La Rocca R, et al. Lipid droplets: a new player in colorectal cancer stem cells unveiled by spectroscopic imaging. Stem cells. 2015;33(1):35–44.
Liu M, Xia Y, Ding J, Ye B, Zhao E, Choi J-H, et al. Transcriptional profiling reveals a common metabolic program in high-risk human neuroblastoma and mouse neuroblastoma sphere-forming cells. Cell Rep. 2016;17(2):609–23.
Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 2014;15(4):243–56.
Chen C-L, Uthaya Kumar Dinesh B, Punj V, Xu J, Sher L, Tahara Stanley M, et al. NANOG metabolically reprograms tumor-initiating stem-like cells through tumorigenic changes in oxidative phosphorylation and fatty acid metabolism. Cell Metab. 2016;23(1):206–19.
Dianat-Moghadam H, Mahari A, Heidarifard M, Parnianfard N, Pourmousavi-Kh L, Rahbarghazi R, et al. NK cells-directed therapies target circulating tumor cells and metastasis. Cancer Lett. 2021;497:41–53.
Wu G, Wang Q, Xu Y, Li J, Zhang H, Qi G, et al. Targeting the transcription factor receptor LXR to treat clear cell renal cell carcinoma: agonist or inverse agonist? Cell Death Dis. 2019;10(6):416.
Connor JH, Weiser DC, Li S, Hallenbeck JM, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol Cell Biol. 2001;21(20):6841–50.
Ariyama H, Kono N, Matsuda S, Inoue T, Arai H. Decrease in membrane phospholipid unsaturation induces unfolded protein response*. J Biol Chem. 2010;285(29):22027–35.
Acknowledgements
The authors appreciate the personnel of Drug Applied Research Center for help and guide.
Funding
This work was financially supported by Tabriz University of Medical Sciences (Hassan Dianat-Moghadam Ph.D. Thesis. Approval ID: IR.TBZMED.VCR.REC.1397.097).
Author information
Authors and Affiliations
Contributions
HDM, and SAR performed experiments and prepared draft. MN, RR, and HH designed and supervised the study. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that there is no conflict of interest.
Ethical approval
Not applicable.
Consent for publication
All authors agree with the publication of this manuscript in this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dianat-Moghadam, H., Abbasspour-Ravasjani, S., Hamishehkar, H. et al. LXR inhibitor SR9243-loaded immunoliposomes modulate lipid metabolism and stemness in colorectal cancer cells. Med Oncol 40, 156 (2023). https://doi.org/10.1007/s12032-023-02027-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02027-4