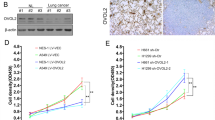
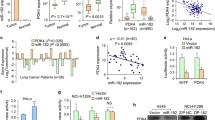
Abstract
Metabolic reprogramming is considered one of the main driving forces for tumor progression, providing energy and substrates of biosynthesis to support rapid neoplastic proliferation. Particularly, the tumor suppressor protein p53 was shown to revert the Warburg effect and play complex roles in regulating glucose metabolism. Jumonji C domain-containing protein 5 (JMJD5) has previously been reported as a negative regulator of p53. However, the role of JMJD5 in p53-mediated metabolic reprogramming remains elusive. Here, we discovered that knockdown of JMJD5 significantly enhances TIGAR expression in p53 wild-type non-small cell lung cancer (NSCLC) cells, which could further suppress glycolysis and promote the pentose phosphate pathway. Besides, JMJD5 knockdown promotes the NSCLC cell proliferation in vitro and xenograft tumor growth in vivo, while silencing TIGAR can abolish this effect. Low JMJD5 expression levels are associated with elevated TIGAR levels and correlates with poor prognosis in lung cancer patients. Taken together, our findings suggest that JMJD5 is a key regulator of tumor glucose metabolism by targeting the p53/TIGAR metabolic pathway.





Similar content being viewed by others
Data availability
All the datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. https://doi.org/10.1158/2159-8290.CD-21-1059.
Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. https://doi.org/10.1126/science.123.3191.309.
Gomes AS, et al. p53 and glucose metabolism: an orchestra to be directed in cancer therapy. Pharmacol Res. 2018;131:75–86. https://doi.org/10.1016/j.phrs.2018.03.015.
Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci. 2014;39(8):347–54. https://doi.org/10.1016/j.tibs.2014.06.005.
Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative Stress in Cancer. Cancer Cell. 2020;38(2):167–97. https://doi.org/10.1016/j.ccell.2020.06.001.
Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137(3):413–31. https://doi.org/10.1016/j.cell.2009.04.037.
Mello SS, Attardi LD. Deciphering p53 signaling in tumor suppression. Curr Opin Cell Biol. 2018;51:65–72. https://doi.org/10.1016/j.ceb.2017.11.005.
Liu J, et al. Tumor suppressor p53 and metabolism. J Mol Cell Biol. 2019;11(4):284–92. https://doi.org/10.1093/jmcb/mjy070.
Liu Y, Gu W. The complexity of p53-mediated metabolic regulation in tumor suppression. Semin Cancer Biol. 2022;85:4–32. https://doi.org/10.1016/j.semcancer.2021.03.010.
Oh S, Janknecht R. Histone demethylase JMJD5 is essential for embryonic development. Biochem Biophys Res Commun. 2012;420(1):61–5. https://doi.org/10.1016/j.bbrc.2012.02.115.
Ishimura A, et al. Jmjd5, an H3K36me2 histone demethylase, modulates embryonic cell proliferation through the regulation of Cdkn1a expression. Development. 2012;139(4):749–59. https://doi.org/10.1242/dev.074138.
Zhu H, Hu S, Baker J. JMJD5 regulates cell cycle and pluripotency in human embryonic stem cells. Stem Cells. 2014;32(8):2098–110. https://doi.org/10.1002/stem.1724.
Youn MY, et al. JMJD5, a Jumonji C (JmjC) domain-containing protein, negatively regulates osteoclastogenesis by facilitating NFATc1 protein degradation. J Biol Chem. 2012;287(16):12994–3004. https://doi.org/10.1074/jbc.M111.323105.
Jones MA, et al. Jumonji domain protein JMJD5 functions in both the plant and human circadian systems. Proc Natl Acad Sci U S A. 2010;107(50):21623–8. https://doi.org/10.1073/pnas.1014204108.
Saran AR, et al. JMJD5 links CRY1 function and proteasomal degradation. PLoS Biol. 2018;16(11):e2006145. https://doi.org/10.1371/journal.pbio.2006145.
Zhao Z, et al. Overexpression of histone demethylase JMJD5 promotes metastasis and indicates a poor prognosis in breast cancer. Int J Clin Exp Pathol. 2015;8(9):10325–34.
Wang HJ, et al. JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1alpha-mediated glucose metabolism. Proc Natl Acad Sci U S A. 2014;111(1):279–84. https://doi.org/10.1073/pnas.1311249111.
Wang HJ, et al. KDM8/JMJD5 as a dual coactivator of AR and PKM2 integrates AR/EZH2 network and tumor metabolism in CRPC. Oncogene. 2019;38(1):17–32. https://doi.org/10.1038/s41388-018-0414-x.
Zhang R, et al. JMJD5 is a potential oncogene for colon carcinogenesis. Int J Clin Exp Pathol. 2015;8(6):6482–9.
Yao Y, Zhou WY, He RX. Down-regulation of JMJD5 suppresses metastasis and induces apoptosis in oral squamous cell carcinoma by regulating p53/NF-kappaB pathway. Biomed Pharmacother. 2019;109:1994–2004. https://doi.org/10.1016/j.biopha.2018.07.144.
Song J, et al. A novel protein encoded by ZCRB1-induced circHEATR5B suppresses aerobic glycolysis of GBM through phosphorylation of JMJD5. J Exp Clin Cancer Res. 2022;41(1):171. https://doi.org/10.1186/s13046-022-02374-6.
Qu LH, et al. Comprehensive analyses of prognostic biomarkers and immune infiltrates among histone lysine demethylases (KDMs) in hepatocellular carcinoma. Cancer Immunol Immunother. 2022;71(10):2449–67. https://doi.org/10.1007/s00262-022-03167-8.
Wang Z, et al. Differential proteome profiling of pleural effusions from lung cancer and benign inflammatory disease patients. Biochim Biophys Acta. 2012;1824(4):692–700. https://doi.org/10.1016/j.bbapap.2012.01.016.
Oh S, Shin S, Janknecht R. The small members of the JMJD protein family: enzymatic jewels or jinxes? Biochim Biophys Acta Rev Cancer. 2019;1871(2):406–18. https://doi.org/10.1016/j.bbcan.2019.04.002.
Wang H, et al. Jumonji-C domain-containing protein 5 suppresses proliferation and aerobic glycolysis in pancreatic cancer cells in a c-Myc-dependent manner. Cell Signal. 2022;93:110282. https://doi.org/10.1016/j.cellsig.2022.110282.
Huang X, et al. JMJD5 interacts with p53 and negatively regulates p53 function in control of cell cycle and proliferation. Biochim Biophys Acta. 2015;1853(10 pt A):2286–95. https://doi.org/10.1016/j.bbamcr.2015.05.026.
Bensaad K, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126(1):107–20. https://doi.org/10.1016/j.cell.2006.05.036.
Green DR, Chipuk JE. p53 and metabolism: inside the TIGAR. Cell. 2006;126(1):30–2. https://doi.org/10.1016/j.cell.2006.06.032.
Tang J, et al. Structure, regulation, and biological functions of TIGAR and its role in diseases. Acta Pharmacol Sin. 2021;42(10):1547–55. https://doi.org/10.1038/s41401-020-00588-y.
Liu H, et al. Clipping of arginine-methylated histone tails by JMJD5 and JMJD7. Proc Natl Acad Sci U S A. 2017;114(37):E7717–26. https://doi.org/10.1073/pnas.1706831114.
Shen J, et al. JMJD5 cleaves monomethylated histone H3 N-tail under DNA damaging stress. EMBO Rep. 2017;18(12):2131–43. https://doi.org/10.15252/embr.201743892.
Wilkins SE, et al. JMJD5 is a human arginyl C-3 hydroxylase. Nat Commun. 2018;9(1):1180. https://doi.org/10.1038/s41467-018-03410-w.
Berkers CR, et al. Metabolic regulation by p53 family members. Cell Metab. 2013;18(5):617–33. https://doi.org/10.1016/j.cmet.2013.06.019.
Maddocks OD, et al. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 2013;493(7433):542–6. https://doi.org/10.1038/nature11743.
Cheung EC, Ludwig RL, Vousden KH. Mitochondrial localization of TIGAR under hypoxia stimulates HK2 and lowers ROS and cell death. Proc Natl Acad Sci U S A. 2012;109(50):20491–6. https://doi.org/10.1073/pnas.1206530109.
Geng J, et al. The diverse role of TIGAR in cellular homeostasis and cancer. Free Radic Res. 2018;52(11–12):1240–9. https://doi.org/10.1080/10715762.2018.1489133.
Li M, et al. A TIGAR-regulated metabolic pathway is critical for protection of brain ischemia. J Neurosci. 2014;34(22):7458–71. https://doi.org/10.1523/JNEUROSCI.4655-13.2014.
Wang J, et al. Reprogramming metabolism by histone methyltransferase NSD2 drives endocrine resistance via coordinated activation of pentose phosphate pathway enzymes. Cancer Lett. 2016;378(2):69–79. https://doi.org/10.1016/j.canlet.2016.05.004.
Xie JM, et al. TIGAR has a dual role in cancer cell survival through regulating apoptosis and autophagy. Cancer Res. 2014;74(18):5127–38. https://doi.org/10.1158/0008-5472.CAN-13-3517.
Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. https://doi.org/10.3322/caac.21708.
Donehower LA, et al. Integrated analysis of TP53 gene and pathway alterations in the cancer genome Atlas. Cell Rep. 2019;28(5):1370-84 e5. https://doi.org/10.1016/j.celrep.2019.07.001.
Funding
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (LY20H160040 and LY18H160024) and the National Natural Science Foundation of China (81772919).
Author information
Authors and Affiliations
Contributions
All authors have contributed to this study. SJ, QH, LG: experimental design, material preparation, data collection and analysis; SJ, LG: writing the first draft of the manuscript; SJ: funding acquisition. All authors commented on previous versions, and read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, G., Qi, H. & Shen, J. JMJD5 inhibits lung cancer progression by regulating glucose metabolism through the p53/TIGAR pathway. Med Oncol 40, 145 (2023). https://doi.org/10.1007/s12032-023-02016-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02016-7