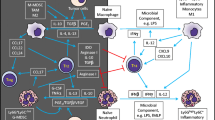
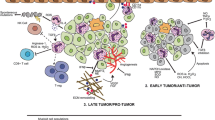
Abstract
Neutrophils are the predominant white blood cells (WBC) that are recruited to the sites of inflammation and infection. They are acknowledged to perform dual roles by promoting (pro-tumor) or by exhibiting anti-cancer properties (anti-tumor). Neutrophils are characterized based on the changes in phenotype and functional properties. To this context, circulating polymorphonuclear neutrophils (cPMN) and tumor-associated neutrophils (TANs) in cancer biology has been well explored but limited to oral polymorphonuclear neutrophils (oPMNs) in oral squamous cell carcinoma (OSCC). However, oPMNs are eminent in maintaining the healthy oral ecosystem by neutralizing microorganisms. Neutralization process enhances the expression of cell surface markers (CD11b, CD63, CD66, CD66b, CD66c, and CD66e) and inflammatory cytokines (TNF-α, IFN-γ, GM-CSF, and IL-8) and increases the recruitment of neutrophils. Along with the inflammation, it has been reported that CEACAM1 and chemerin also favors the infiltration of neutrophils to the cancer site. This indicates that oPMN might contribute to the aetiology of OSCC. The main objective of this review is to explore, the production and migration of oPMNs to the oral cavity, their phenotypes and possible role in OSCC.




Similar content being viewed by others
Data availability
Data were collected from Scopus, PubMed and goggle scholar.
Abbreviations
- cPMN:
-
Circulating polymorphonuclear neutrophils
- oPMN:
-
Oral polymorphonuclear neutrophils
- IL:
-
Interleukin
- MMP:
-
Matrix metallopeptidase
- CXCL:
-
C-X-C Motif chemokine ligand
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- TNF:
-
Tumor Necrosis Factor
- fMLP:
-
N-formyl-methionyl-leucyl-phenylalanine
- GM-CSF:
-
Granulocyte macrophage colony-stimulating factor
- TIMP:
-
Tissue inhibitor of metalloproteinase
References
Rivera C. Essentials of oral cancer. Int J Clin Exp Pathol. 2015;8(9):11884–94.
Usman S, et al. Major molecular signaling pathways in oral cancer associated with therapeutic resistance. Front Oral Health. 2021. https://doi.org/10.3389/froh.2020.603160.
Siegel R, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29.
Le Campion A, Ribeiro CMB. Low survival rates of oral and oropharyngeal squamous cell carcinoma. Int J Dent. 2017;2017:5815493.
Sathiasekar AC, et al. Oral field cancerization and its clinical implications in the management in potentially malignant disorders. J Pharm Bioallied Sci. 2017;9(Suppl 1):S23-s25.
Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. 2021;14(1):173.
Domnich M, et al. Oral neutrophils: underestimated players in oral cancer. Front Immunol. 2020. https://doi.org/10.3389/fimmu.2020.565683.
Wang N, et al. Neutrophils infiltration in the tongue squamous cell carcinoma and its correlation with CEACAM1 expression on tumor cells. PLoS ONE. 2014;9(2):e89991.
Granot Z. Neutrophils as a therapeutic target in cancer. Front Immunol. 2019;10:1710.
Trellakis S, et al. Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. Int J Cancer. 2011;129(9):2183–93.
Rijkschroeff P, Loos BG, Nicu EA. oral polymorphonuclear neutrophil contributes to oral health. Curr Oral Health Rep. 2018;5(4):211–20.
Kilian M, et al. The oral microbiome—an update for oral healthcare professionals. Br Dent J. 2016;221(10):657–66.
Lenka S, Bhuyan SK, Bhuyan R. Understanding the characteristics of the host genome and microbiome interaction in oral squamous cell carcinoma: a narrative review. Beni-Suef Univ J Basic Appl Sci. 2022;11(1):126.
Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol. 2018;9:113.
Moonen CGJ, et al. Oral neutrophils characterized: chemotactic, phagocytic, and neutrophil extracellular trap (NET) formation properties. Front Immunol. 2019;10:635.
Domnich M, et al. Oral Neutrophils: underestimated players in oral cancer. Front Immunol. 2020;11:565683.
Scott DA, Krauss J. Neutrophils in periodontal inflammation. Front Oral Biol. 2012;15:56–83.
Yamamoto M, Saeki K, Utsumi K. Isolation of human salivary polymorphonuclear leukocytes and their stimulation-coupled responses. Arch Biochem Biophys. 1991;289(1):76–82.
Uribe-Querol E, Rosales C. Neutrophils in cancer: two sides of the same coin. J Immunol Res. 2015;2015:983698.
Martinez FO, et al. IL-8 induces a specific transcriptional profile in human neutrophils: synergism with LPS for IL-1 production. Eur J Immunol. 2004;34(8):2286–92.
Hu X, et al. Neutrophils promote tumor progression in oral squamous cell carcinoma by regulating EMT and JAK2/STAT3 signaling through chemerin. Front Oncol. 2022;12:812044.
Sahibzada HA, et al. Salivary IL-8, IL-6 and TNF-α as potential diagnostic biomarkers for oral cancer. Diagnostics. 2017;7(2):21.
Ferrari E, et al. Salivary cytokines as biomarkers for oral squamous cell carcinoma: a systematic review. Int J Mol Sci. 2021;22(13):6795.
Magalhaes MA, Glogauer JE, Glogauer M. Neutrophils and oral squamous cell carcinoma: lessons learned and future directions. J Leukoc Biol. 2014;96(5):695–702.
Lakschevitz FS, et al. Identification of neutrophil surface marker changes in health and inflammation using high-throughput screening flow cytometry. Exp Cell Res. 2016;342(2):200–9.
Fine N, et al. Distinct oral neutrophil subsets define health and periodontal disease states. J Dent Res. 2016;95(8):931–8.
Hirschfeld J. Neutrophil subsets in periodontal health and disease: a mini review. Front Immunol. 2019;10:3001.
Wu L, et al. Tumor-associated neutrophils in cancer: going pro. Cancers. 2019;11(4):564.
Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–94.
Andzinski L, et al. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int J Cancer. 2016;138(8):1982–93.
Glogauer JE, et al. Neutrophils increase oral squamous cell carcinoma invasion through an invadopodia-dependent pathway. Cancer Immunol Res. 2015;3(11):1218–26.
Sagiv JY, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10(4):562–73.
Jablonska E, et al. VEGF, IL-18 and NO production by neutrophils and their serum levels in patients with oral cavity cancer. Cytokine. 2005;30(3):93–9.
Scheibenbogen C, et al. Serum interleukin-8 (IL-8) is elevated in patients with metastatic melanoma and correlates with tumour load. Melanoma Res. 1995;5(3):179–82.
Schmidt H, et al. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer. 2005;93(3):273–8.
Dumitru CA, et al. Tumor-derived macrophage migration inhibitory factor modulates the biology of head and neck cancer cells via neutrophil activation. Int J Cancer. 2011;129(4):859–69.
Trellakis S, et al. Peripheral blood neutrophil granulocytes from patients with head and neck squamous cell carcinoma functionally differ from their counterparts in healthy donors. Int J Immunopathol Pharmacol. 2011;24(3):683–93.
Decker AS, et al. Prognostic role of blood NETosis in the progression of head and neck cancer. Cells. 2019;8(9):946.
Giese MA, Hind LE. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133(20):2159–67.
Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Gene Dev. 2018;32(19–20):1267–84.
Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–94.
Arvanitakis K, Mitroulis I, Germanidis G. Tumor-associated neutrophils in hepatocellular carcinoma pathogenesis, prognosis, and therapy. Cancers. 2021;13(12):2899.
Masucci MT, Minopoli M, Carriero MV. Tumor associated neutrophils: their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol. 2019;9:1146.
Shaul ME, et al. Tumor-associated neutrophils display a distinct N1 profile following TGFβ modulation: a transcriptomics analysis of pro- vs antitumor TANs. OncoImmunology. 2016;5(11):e1232221.
Ohms M, Möller S, Laskay T. An attempt to polarize human neutrophils toward N1 and N2 phenotypes in vitro. Front Immunol. 2020;11:532.
GómezRomán VR, Murray JC, Weiner LM. Chapter 1—antibody-dependent cellular cytotoxicity (ADCC). In: Ackerman ME, Nimmerjahn F, editors. Antibody Fc. Academic Press: Boston; 2014. p. 1–27.
Singh JK, et al. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 2013;15(4):210.
Güngör N, et al. Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis. 2010;25(2):149–54.
Houghton AM, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010;16(2):219–23.
Das A, et al. MMP proteolytic activity regulates cancer invasiveness by modulating integrins. Sci Rep. 2017;7(1):14219.
Rotondo R, et al. IL-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. Int J Cancer. 2009;125(4):887–93.
Yachimovich-Cohen N, et al. Human embryonic stem cells suppress T cell responses via arginase I-dependent mechanism. J Immunol. 2010;184(3):1300–8.
Yang J, et al. Loss of CXCR4 in myeloid cells enhances antitumor immunity and reduces melanoma growth through NK cell and FASL mechanisms. Cancer Immunol Res. 2018;6(10):1186–98.
Michaeli J, et al. Tumor-associated neutrophils induce apoptosis of non-activated CD8 T-cells in a TNFα and NO-dependent mechanism, promoting a tumor-supportive environment. Oncoimmunology. 2017;6(11):e1356965.
Sun B, et al. Neutrophil suppresses tumor cell proliferation via Fas /Fas ligand pathway mediated cell cycle arrested. Int J Biol Sci. 2018;14(14):2103–13.
Matlung HL, et al. Neutrophils kill antibody-opsonized cancer cells by trogoptosis. Cell Rep. 2018;23(13):3946-3959.e6.
Lakschevitz FS, Aboodi GM, Glogauer M. Oral neutrophils display a site-specific phenotype characterized by expression of T-cell receptors. J Periodontol. 2013;84(10):1493–503.
Brekke OL, et al. The role of complement C3 opsonization, C5a receptor, and CD14 in E coli-induced up-regulation of granulocyte and monocyte CD11b/CD18 (CR3), phagocytosis, and oxidative burst in human whole blood. J Leukoc Biol. 2007;81(6):1404–13.
Kuijpers TW, et al. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood. 1991;78(4):1105–11.
Irani S, Barati I, Badiei M. Periodontitis and oral cancer—current concepts of the etiopathogenesis. Oncol Rev. 2020;14(1):465.
Elebyary O, et al. The crossroads of periodontitis and oral squamous cell carcinoma: immune implications and tumor promoting capacities. Front Oral Health. 2021. https://doi.org/10.3389/froh.2020.584705.
Tomita M, et al. Preoperative neutrophil to lymphocyte ratio as a prognostic predictor after curative resection for non-small cell lung cancer. Anticancer Res. 2011;31(9):2995–8.
Singh S, et al. Diagnostic efficacy of neutrophil to lymphocyte ratio (NLR) in oral potentially malignant disorders and oral cancer. Indian J Pathol Microbiol. 2021;64(2):243–9.
Bhuyan R, et al. Periodontitis and its inflammatory changes linked to various systemic diseases: a review of its underlying mechanisms. Biomedicines. 2022;10(10):2659.
Laliberté C, et al. Characterization of oral squamous cell carcinoma associated inflammation: a pilot study. Front Oral Health. 2021;2:740469.
Acknowledgements
The authors would like to express their gratitude to the president of Siksha ‘O’ Anusandhan (deemed to be university), India for giving the required facilities to carry out the aforementioned study.
Funding
None.
Author information
Authors and Affiliations
Contributions
SL performed literature searches and wrote the manuscript. RB and RKB contributed to the concept and reviewed the manuscript. PRV and SB edited and designed the manuscript. All authors contributed to manuscript revision, and approved the submitted version.
Corresponding author
Ethics declarations
Competing interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lenka, S., Bhola, R.K., Varanasi, P.R. et al. Understanding the functional relevance of oral neutrophils, phenotype and properties in OSCC. Med Oncol 40, 134 (2023). https://doi.org/10.1007/s12032-023-02010-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02010-z