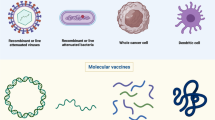
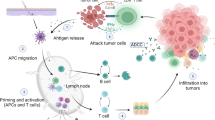
Abstract
Cancer immunotherapy is one of the recently developed cancer treatment modalities. When compared with conventional anticancer drug regimens, immunotherapy has shown significantly better outcomes in terms of quality of life and overall survival. It incorporates a wide range of immunomodulatory modalities that channel the effects of the immune system either by broadly modulating the host immune system or by accurately targeting distinct tumor antigens. One such treatment modality that has gained interest is cancer vaccine therapy which acts by developing antibodies against tumor cells. Cancer vaccines target individual peptides or groups of antigens that are released by tumor cells and presented by the APCs. This also initiates an effective process to activate the host immune responses. Studies on various types of cancer vaccines are conducted, out of which only few are approved by FDA for clinical uses. Despite of documented safety and efficacy of conventional chemotherapy and cancer vaccines, individually they did not produce substantial results in eradication of the cancer as a monotherapy. Hence, the combination approach holds the extensive potential to provide significant improvement in disease outcomes. Certain chemotherapy has immunomodulatory effects and is proven to synergize with cancer vaccines thereby enhancing their anti-tumor activities. Chemotherapeutic agents are known to have immunostimulatory mechanisms apart from its cytotoxic effect and intensify the anti-tumor activities of vaccines by various mechanisms. This review highlights various cancer vaccines, their mechanism, and how their activity gets affected by chemotherapeutic agents. It also aims at summarizing the evidence-based outcome of the combination approach of a cancer vaccine with chemotherapy and a brief on future aspects.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AEs:
-
: Adverse events
- APCs:
-
: Antigen-presenting cells
- ATP:
-
: Adenosine triphosphate
- B7-H3:
-
: B7 Homolog 3
- B7-H4:
-
: B7 Homolog 4
- CBR:
-
: Clinical Benefit Rate
- CD19:
-
: Cluster of Differentiation 19
- CD30:
-
: Cluster of Differentiation 30
- CD40:
-
: Cluster of differentiation 40
- CD70:
-
: Cluster of Differentiation 70
- CD8 + :
-
: Cluster of differentiation 8
- CDKs:
-
: Cyclin-dependent kinases
- CEA:
-
: Carcinoembryonic antigen
- cGMP:
-
: Guanosine 3',5'-cyclic monophosphate
- CRC:
-
: Colorectal cancer
- CT83:
-
: Cancer/Testis Antigen 83
- CTLA-4:
-
: Cytotoxic T lymphocyte associated protein 4
- CY:
-
: Cyclophosphamide
- DAMPs:
-
: Damage-associated molecular patterns
- DCs:
-
: Dendritic cells
- DFRS:
-
: Distant recurrence-free survival
- DFS:
-
: Disease-free survival
- DLTs:
-
: Dose-limiting toxicities
- DNA:
-
: Deoxyribonucleic acid
- EGFR:
-
: Epidermal growth factor receptor
- GI:
-
: Gastrointestinal
- GM-CSF:
-
: Granulocyte–macrophage colony-stimulating factor
- GP100:
-
: Glycoprotein 100
- HCV:
-
: Hepatitis C virus
- HER2:
-
: Human epidermal growth factor 2
- HLA:
-
: Human leukocyte antigen
- HMGB1:
-
: High mobility group box 1 protein
- HPV 16/17:
-
: Human papillomavirus 16/17
- ICD:
-
: Immunologic cell death
- iDFS:
-
: Invasive disease-free survival
- IDO:
-
: Indoleamine-2,3-dioxygenase
- IFN-α:
-
: Interferons-alpha
- IFN-γ:
-
: Interferon‐gamma
- IGF1R:
-
: Insulin-like growth factor 1 receptor
- IMRT:
-
: Intensity-modulated radiotherapy
- IR:
-
: Immunological response
- LAG3:
-
: Lymphocyte Activation Gene 3
- LCK:
-
: Lymphocyte-specific protein tyrosine kinase
- LPA1:
-
: Lysophosphatidic acid
- MAGEA1-40:
-
: Melanoma-associated antigen
- MBC:
-
: Metastatic Breast Cancer
- MDSCs:
-
: Myeloid-derived suppressor cells
- mFOLFOX6:
-
: Combined chemotherapy including leucovorin calcium (folinic acid), fluorouracil, and oxaliplatin
- MHC-II:
-
: Major histocompatibility complex class II
- MTD:
-
: Maximum tolerated dose
- MUC1:
-
: Mucin 1
- NCOA1:
-
: Nuclear receptor co-activator
- NK cells:
-
: Natural killer cells
- NYSEO 1:
-
: New York oesophageal squamous cell carcinoma
- ORR:
-
: Objective response rate
- OS:
-
: Overall survival
- pCR:
-
: Pathological complete response
- PCR:
-
: Polymerase chain reaction
- PD1/PDL1:
-
: Programmed death ligand- 1
- PEP-3:
-
: Epidermal growth factor receptor variant III (EGRRvIII)- specific peptide
- PSA:
-
: Prostate stimulating antigen
- PSA:
-
: Prostate-specific antigen
- RFS:
-
: Recurrence-free survival
- RLRs:
-
: RIG-I-like Receptors
- SBRT:
-
: Stereotactic Body Radiation
- SKILS:
-
: Skin-test infiltrating lymphocytes
- SLP:
-
: Synthetic long peptide
- Sp17:
-
: Sperm protein 17
- STAT1:
-
: Signal transducer, and activator of transcription 1
- STAT3:
-
: Signal transducer and activator of transcription 3
- STINGs:
-
: Stimulator of Interferon Genes
- TAA:
-
: Tumor-associated antigens
- TC-1:
-
: T -cytotoxic 1
- TCR:
-
: T-cell receptor
- TCRA:
-
: T-cell receptor alpha
- TCRD:
-
: T Cell Receptor Delta Locus
- TCRG:
-
: T-cell Receptor Gamma
- TGF-β:
-
: Transforming growth factor-β
- TLRs:
-
: Toll-like Receptors
- TME:
-
: Tumor microenvironment
- TNBC:
-
: Triple-negative breast cancer
- TNF:
-
: Tumor necrosis factor
- Tregs:
-
: Regulatory T-cells
- TRICOM:
-
: Three human T-cell costimulatory molecules
- TTP:
-
: Time to progression
- VSV:
-
: Vesicular stomatitis virus
- WT-1:
-
: Wilms' tumor suppressor gene1
References
Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutr Metab. 2010;7(1):1–22. https://doi.org/10.1186/1743-7075-7-7.
McCormick PJ. Cancer tsunami: emerging trends, economic burden, and perioperative implications. Curr Anesthesiol Rep. 2018;8(4):348–54. https://doi.org/10.1007/s40140-018-0294-1.
Popat K, McQueen K, Feeley TW. The global burden of cancer. Best Pract Res Clin Anaesthesiol. 2013;27(4):399–408. https://doi.org/10.1016/j.bpa.2013.10.010.
Cancer [Internet]. World Health Organization. . Available from: https://www.who.int/health-topics/cancer#tab=tab_1.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
INDIA GLOBOCAN-2020: 365 India-fact-sheets: the global cancer observatory, WHO; 2021 [Available from: https://gco.iarc.fr/today/data/factsheets/populations/356-india-fact-sheets.pdf.
Sahoo SS, Verma M, Parija pp. An overview of cancer registration in India: present status and future challenges. Oncol J India. 2018;2(4):86. https://doi.org/10.4103/oji.oji_40_18.
Sivaram S, Majumdar G, Perin D, Nessa A, Broeders M, Lynge E, et al. Population-based cancer screening programmes in low-income and middle-income countries: regional consultation of the International Cancer Screening Network in India. Lancet Oncol. 2018;19(2):e113–22. https://doi.org/10.1016/S1470-2045(18)30003-2.
Chalkidou K, Marquez P, Dhillon PK, Teerawattananon Y, Anothaisintawee T, Gadelha CAG, et al. Evidence-informed frameworks for cost-effective cancer care and prevention in low, middle, and high-income countries. Lancet Oncol. 2014;15(3):e119–31. https://doi.org/10.1016/S1470-2045(13)70547-3.
Takiar R, Nadayil D, Nandakumar A. Projections of number of cancer cases in India (2010–2020) by cancer groups. J Asian Pac J Cancer Prev. 2010;11(4):1045–9.
Baxevanis CN, Perez SA, Papamichail M. Combinatorial treatments including vaccines, chemotherapy and monoclonal antibodies for cancer therapy. Cancer Immunol Immunother. 2009;58(3):317–24. https://doi.org/10.1007/s00262-008-0576-4.
Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin. 2012;62(5):309–35. https://doi.org/10.3322/caac.20132.
Borghaei H, Smith MR, Campbell KS. Immunotherapy of cancer. Eur J Pharmacol. 2009;625(1–3):41–54. https://doi.org/10.1016/j.ejphar.2009.09.067.
Corrie PG. Cytotoxic chemotherapy: clinical aspects. J Med. 2008;36(1):24–8. https://doi.org/10.1016/j.mpmed.2011.09.012.
Ventola CL. Cancer immunotherapy, part 1: current strategies and agents. Pharmacy Ther. 2017;42(6):375.
Alatrash G, Jakher H, Stafford PD, Mittendorf EA. Cancer immunotherapies, their safety and toxicity. Expert Opin Drug Saf. 2013;12(5):631–45. https://doi.org/10.1517/14740338.2013.795944.
Forni G. Vaccines for tumor prevention: a pipe dream? J Infect Dev Ctries. 2015;9(06):600–8. https://doi.org/10.3855/jidc.7201.
Olivieri B, Betterle C, Zanoni G. Vaccinations and autoimmune diseases. Vaccines. 2021;9(8):815. https://doi.org/10.3390/vaccines9080815.
Bereau M. Vaccination and induction of autoimmune diseases. Inflamm Allergy Drug Targets. 2015;14(2):94–8. https://doi.org/10.2174/1871528114666160105113046.
Mocellin S, Mandruzzato S, Bronte V, Lise M, Nitti D. Part I: vaccines for solid tumours. Lancet Oncol. 2004;5(11):681–9. https://doi.org/10.1016/S1470-2045(04)01610-9.
Choudhury A, Mosolits S, Kokhaei P, Hansson L, Palma M, Mellstedt H. Clinical results of vaccine therapy for cancer: learning from history for improving the future. Adv Cancer Res. 2006;95:147–202. https://doi.org/10.1016/S0065-230X(06)95005-2.
Hodge JW, Ardiani A, Farsaci B, Kwilas AR, Gameiro SR. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. In: Hodge JW, Ardiani A, Farsaci B, Kwilas AR, Gameiro SR, editors. Seminars in oncology. Amsterdam: Elsevier; 2012.
Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17(12):725–41. https://doi.org/10.1038/s41571-020-0413-z.
Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of anti-cancer chemotherapy. Nat Rev Immunol. 2007. https://doi.org/10.1038/nri2216.
Shurin MR, Naiditch H, Gutkin DW, Umansky V, Shurin GV. ChemoImmunoModulation: immune regulation by the antineoplastic chemotherapeutic agents. Curr Med Chem. 2012;19(12):1792–803. https://doi.org/10.2174/092986712800099785.
Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28(6):690–714. https://doi.org/10.1016/j.ccell.2015.10.012.
Tanaka A, Nishikawa H, Noguchi S, Sugiyama D, Morikawa H, Takeuchi Y, et al. Tyrosine kinase inhibitor imatinib augments tumor immunity by depleting effector regulatory T cells. J Exp Med. 2019;217(2):e20191009. https://doi.org/10.1084/jem.20191009.
Rozkova D, Horvath R, Bartunkova J, Spisek R. Glucocorticoids severely impair differentiation and antigen presenting function of dendritic cells despite upregulation of Toll-like receptors. Clin Immunol. 2006;120(3):260–71. https://doi.org/10.1016/j.clim.2006.04.567.
Seiwert TY, Haddad RI, Gupta S, Mehra R, Tahara M, Berger R, et al. Antitumor activity and safety of pembrolizumab in patients (pts) with advanced squamous cell carcinoma of the head and neck (SCCHN): preliminary results from KEYNOTE-012 expansion cohort. Proc Am Soc Clin Oncol. 2015. https://doi.org/10.1200/jco.2015.33.18_suppl.lba6008.
Claude L, Perol D, Ray-Coquard I, Petit T, Blay J-Y, Carrie C, et al. Lymphopenia: a new independent prognostic factor for survival in patients treated with whole brain radiotherapy for brain metastases from breast carcinoma. Radiat Oncol. 2005;76(3):334–9. https://doi.org/10.1016/j.radonc.2005.06.004.
Weiner H, Cohen J. Treatment of multiple sclerosis with cyclophosphamide: critical review of clinical and immunologic effects. Mult Scler. 2002;8(2):142–54. https://doi.org/10.1191/1352458502ms790oa.
Panici PB, Maggioni A, Hacker N, Landoni F, Ackermann S, Campagnutta E, et al. Systematic aortic and pelvic lymphadenectomy versus resection of bulky nodes only in optimally debulked advanced ovarian cancer: a randomized clinical trial. J Natl Cancer Inst. 2005;97(8):560–6. https://doi.org/10.1093/jnci/dji102.
Zhang J, Pan S, Jian C, Hao L, Dong J, Sun Q, et al. Immunostimulatory properties of chemotherapy in breast cancer: from immunogenic modulation mechanisms to clinical practice. Front immunol. 2022;12:5711. https://doi.org/10.3389/fimmu.2021.819405.
Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31(1):51–72. https://doi.org/10.1146/annurev-immunol-032712-100008.
Garg AD, More S, Rufo N, Mece O, Sassano ML, Agostinis P, et al. Trial watch: immunogenic cell death induction by anticancer chemotherapeutics. OncoImmunology. 2017;6(12):e1386829. https://doi.org/10.1080/2162402X.2017.1386829.
Zhou J, Wang G, Chen Y, Wang H, Hua Y, Cai Z. Immunogenic cell death in cancer therapy: present and emerging inducers. J Cell Mol Med. 2019;23(8):4854–65. https://doi.org/10.1111/jcmm.14356.
Daniel D, Crawford J. Myelotoxicity from chemotherapy. In: Daniel D, Crawford J, editors. Seminars in oncology. Amsterdam: Elsevier; 2006.
Barreto JN, McCullough KB, Ice LL, Smith JA. Antineoplastic agents and the associated myelosuppressive effects: a review. J Pharm Pract. 2014;27(5):440–6. https://doi.org/10.1177/0897190014546108.
Wumkes M, Van Der Velden A, Los M, Leys M, Beeker A, Nijziel M, et al. Serum antibody response to influenza virus vaccination during chemotherapy treatment in adult patients with solid tumours. Vaccine. 2013;31(52):6177–84. https://doi.org/10.1016/j.vaccine.2013.10.053.
Zahid KR, Raza U, Tumbath S, Jiang L, Xu W, Huang X. Neutrophils: musketeers against immunotherapy. Front Oncol. 2022;12:975981. https://doi.org/10.3389/fonc.2022.975981.
Yang D, Liu J. Neutrophil extracellular traps: a new player in cancer metastasis and therapeutic target. J Exp Clin Cancer Res: CR. 2021;40(1):233. https://doi.org/10.1186/s13046-021-02013-6.
Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci USA. 2012;109(32):13076–81. https://doi.org/10.1073/pnas.1200419109.
Arelaki S, Arampatzioglou A, Kambas K, Papagoras C, Miltiades P, Angelidou I, et al. Gradient infiltration of neutrophil extracellular traps in colon cancer and evidence for their involvement in tumour growth. PLoS ONE. 2016;11(5):e0154484. https://doi.org/10.1371/journal.pone.0154484.
Millrud CR, Kågedal Å, Kumlien Georén S, Winqvist O, Uddman R, Razavi R, et al. NET-producing CD16(high) CD62L(dim) neutrophils migrate to tumor sites and predict improved survival in patients with HNSCC. Int J Cancer. 2017;140(11):2557–67. https://doi.org/10.1002/ijc.30671.
Dees S, Ganesan R, Singh S, Grewal IS. Regulatory T cell targeting in cancer: emerging strategies in immunotherapy. Eur J Immunol. 2021;51(2):280–91. https://doi.org/10.1002/eji.202048992.
Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27(1):109–18. https://doi.org/10.1038/cr.2016.151.
Wing JB, Tanaka A, Sakaguchi S. Human FOXP3+ regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity. 2019;50(2):302–16. https://doi.org/10.1016/j.immuni.2019.01.020.
Wang W-H, Xu H-Y, Zhao Z-M, Zhang G-M, Lin F-W. Dynamic and significant changes of T-cell subgroups in breast cancer patients during surgery and chemotherapy. Int Immunopharmacol. 2018;65:279–83. https://doi.org/10.1016/j.intimp.2018.09.039.
Oshi M, Asaoka M, Tokumaru Y, Angarita FA, Yan L, Matsuyama R, et al. Abundance of regulatory T cell (Treg) as a predictive biomarker for neoadjuvant chemotherapy in triple-negative breast cancer. Cancers. 2020;12(10):3038. https://doi.org/10.3390/cancers12103038.
Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol. 2021;21(8):485–98. https://doi.org/10.1038/s41577-020-00490-y.
Safarzadeh E, Mohammadi A, Mansoori B, Duijf PH, Hashemzadeh S, Khaze V, et al. STAT3 silencing and TLR7/8 pathway activation repolarize and suppress myeloid-derived suppressor cells from breast cancer patients. Front immunol. 2021;11:613215. https://doi.org/10.3389/fimmu.2020.613215.
Zhang H, Houghton AM. Good cops turn bad: the contribution of neutrophils to immune-checkpoint inhibitor treatment failures in cancer. Pharmacol Ther. 2021;217:107662. https://doi.org/10.1016/j.pharmthera.2020.107662.
Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non–small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30(17):2046–54. https://doi.org/10.1200/JCO.2011.38.4032.
Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24(1):75–83. https://doi.org/10.1093/annonc/mds213.
Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, Smyth MJ. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res. 2011;71(14):4809–20. https://doi.org/10.1158/0008-5472.CAN-11-0753.
Kuczma M, Ding Z-C, Zhou G. Immunostimulatory effects of melphalan and usefulness in adoptive cell therapy with antitumor CD4+ T cells. Crit Rev Immunol. 2016. https://doi.org/10.1615/CritRevImmunol.2016017507.
Yang H, Antoine DJ, Andersson U, Tracey KJ. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol. 2013;93(6):865–73. https://doi.org/10.1189/jlb.1212662.
Chavez-Dominguez RL, Perez-Medina MA, Lopez-Gonzalez JS, Galicia-Velasco M, Matias-Florentino M, Avila-Rios S, et al. Role of HMGB1 in cisplatin-persistent lung adenocarcinoma cell lines. Front Oncol. 2021;11:750677. https://doi.org/10.3389/fonc.2021.750677.
Martins I, Tesniere A, Kepp O, Michaud M, Schlemmer F, Senovilla L, et al. Chemotherapy induces ATP release from tumor cells. Cell cycle (Georgetown, Tex). 2009;8(22):3723–8. https://doi.org/10.4161/cc.8.22.10026.
Rufo N, Garg AD, Agostinis P. The unfolded protein response in immunogenic cell death and cancer immunotherapy. Trends cancer. 2017;3(9):643–58. https://doi.org/10.1016/j.trecan.2017.07.002.
Hao Y-B, Yi S-Y, Ruan J, Zhao L, Nan K-J. New insights into metronomic chemotherapy-induced immunoregulation. Cancer Lett. 2014;354(2):220–6. https://doi.org/10.1016/j.canlet.2014.08.028.
Maiti R. Metronomic chemotherapy. J Pharmacol Pharmacother. 2014;5(3):186–92. https://doi.org/10.4103/0976-500X.136098.
Cazzaniga ME, Cordani N, Capici S, Cogliati V, Riva F, Cerrito MG. Metronomic chemotherapy. Cancers. 2021;13(9):2236. https://doi.org/10.3390/cancers13092236.
Weinmann H. Cancer immunotherapy: selected targets and small-molecule modulators. ChemMedChem. 2016;11(5):450–66. https://doi.org/10.1002/cmdc.201500566.
Burugu S, Dancsok AR, Nielsen TO. Emerging targets in cancer immunotherapy. In: Burugu S, Dancsok AR, Nielsen TO, editors. Seminars in cancer biology. Amsterdam: Elsevier; 2018.
Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14(8):561–84. https://doi.org/10.1038/nrd4591.
Wang F, Wang S, Zhou Q. The resistance mechanisms of lung cancer immunotherapy. Front Oncol. 2020;10:568059. https://doi.org/10.3389/fonc.2020.568059.
West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924–37. https://doi.org/10.1016/S1470-2045(19)30167-6.
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. https://doi.org/10.1056/NEJMoa1801005.
Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–9. https://doi.org/10.1056/NEJMoa1809064.
Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3(5):436–43. https://doi.org/10.1158/2326-6066.CIR-15-0064.
Chen G, Emens LA. Chemoimmunotherapy: reengineering tumor immunity. Cancer Immunol Immunother. 2013;62(2):203–16. https://doi.org/10.1007/s00262-012-1388-0.
Tang K, Wu Y-H, Song Y, Yu B. Indoleamine 2, 3-dioxygenase 1 (IDO1) inhibitors in clinical trials for cancer immunotherapy. J Hematol Oncol. 2021;14(1):1–21. https://doi.org/10.1186/s13045-021-01080-8.
Li K, Qu S, Chen X, Wu Q, Shi M. Promising targets for cancer immunotherapy: TLRs, RLRs, and STING-mediated innate immune pathways. Int J Mol Sci. 2017;18(2):404. https://doi.org/10.3390/ijms18020404.
Yu L-Y, Tang J, Zhang C-M, Zeng W-J, Yan H, Li M-P, et al. New immunotherapy strategies in breast cancer. Int J Environ Res Public Health. 2017;14(1):68. https://doi.org/10.3390/ijerph14010068.
Schlom J, Hodge JW, Palena C, Tsang K-Y, Jochems C, Greiner JW, et al. Therapeutic cancer vaccines. Adv Cancer Res. 2014;121:67–124. https://doi.org/10.1016/B978-0-12-800249-0.00002-0.
Roselli M, Cereda V, di Bari MG, Formica V, Spila A, Jochems C, et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology. 2013;2(10):e27025. https://doi.org/10.4161/onci.27025.
DeMaria PJ, Bilusic M. Cancer vaccines. Hematol Oncol Clin. 2019;33(2):199–214. https://doi.org/10.1016/j.hoc.2018.12.001.
Zhao R, Lai X. Evolutionary analysis of replicator dynamics about anti-cancer combination therapy. Math Biosci Eng. 2023;20(1):656–82. https://doi.org/10.3934/mbe.2023030.
Dijkgraaf EM, Santegoets SJ, Reyners AK, Goedemans R, Nijman HW, van Poelgeest MI, et al. A phase 1/2 study combining gemcitabine, Pegintron and p53 SLP vaccine in patients with platinum-resistant ovarian cancer. Oncotarget. 2015;6(31):32228. https://doi.org/10.18632/oncotarget.4772.
TP53 gene tumor protein p53 [Available from: https://medlineplus.gov/genetics/gene/tp53/#:~:text=By%20stopping%20cells%20with%20mutated,%22guardian%20of%20the%20genome.%22.
Harrop R, Chu F, Gabrail N, Srinivas S, Blount D, Ferrari A. Vaccination of castration-resistant prostate cancer patients with TroVax (MVA–5T4) in combination with docetaxel: a randomized phase II trial. Cancer Immunol Immunother. 2013;62(9):1511–20. https://doi.org/10.1007/s00262-013-1457-z.
Quoix E, Lena H, Losonczy G, Forget F, Chouaid C, Papai Z, et al. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol. 2016;17(2):212–23. https://doi.org/10.1016/S1470-2045(15)00483-0.
Zheng H, Li M, Wu L, Liu W, Liu Y, Gao J, et al. Progress in the application of hydrogels in immunotherapy of gastrointestinal tumors. Drug Deliv. 2023;30(1):2161670. https://doi.org/10.1080/10717544.2022.2161670.
Welters MJ, van der Sluis TC, van Meir H, Loof NM, van Ham VJ, van Duikeren S, et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci Transl Med. 2016;8(334):334ra52-ra52. https://doi.org/10.1126/scitranslmed.aad8307.
Arlen PM, Madan RA, Hodge JW, Schlom J, Gulley JL. Combining vaccines with conventional therapies for cancer. Update on cancer therapeutics. 2007;2(1):33–9. https://doi.org/10.1016/j.uct.2007.04.004.
Drug Interaction Checker-Medscape [Available from: https://reference.medscape.com/drug-interactionchecker.
Wang ZX, Cao JX, Liu ZP, Cui YX, Li CY, Li D, et al. Combination of chemotherapy and immunotherapy for colon cancer in China: a meta-analysis. World J Gastroenterol. 2014;20(4):1095–06. https://doi.org/10.3748/wjg.v20.i4.1095.
Gameiro SR, Caballero JA, Higgins JP, Apelian D, Hodge JW. Exploitation of differential homeostatic proliferation of T-cell subsets following chemotherapy to enhance the efficacy of vaccine-mediated antitumor responses. Cancer Immunol Immunother. 2011;60(9):1227–42. https://doi.org/10.1007/s00262-011-1020-8.
Julien S, Picco G, Sewell R, Vercoutter-Edouart A, Tarp M, Miles D, et al. Sialyl-Tn vaccine induces antibody-mediated tumour protection in a relevant murine model. Br J Cancer. 2009;100(11):1746–54. https://doi.org/10.1038/sj.bjc.6605083.
Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14(11):3536–44. https://doi.org/10.1158/1078-0432.CCR-07-4025.
Chu Y, Wang L-X, Yang G, Ross HJ, Urba WJ, Prell R, et al. Efficacy of GM-CSF-producing tumor vaccine after docetaxel chemotherapy in mice bearing established Lewis lung carcinoma. J Immunother. 2006;29(4):367–80. https://doi.org/10.1097/01.cji.0000199198.43587.ba.
Eralp Y, Wang X, Wang J-P, Maughan MF, Polo JM, Lachman LB. Doxorubicin and paclitaxel enhance the antitumor efficacy of vaccines directed against HER 2/neuin a murine mammary carcinoma model. Breast Cancer Res. 2004;6(4):1–9. https://doi.org/10.1186/bcr787.
Navashenaq JG, Zamani P, Nikpoor AR, Tavakkol-Afshari J, Jaafari MR. Doxil chemotherapy plus liposomal P5 immunotherapy decreased myeloid-derived suppressor cells in murine model of breast cancer. Nanomedicine. 2020;24:102150. https://doi.org/10.1016/j.nano.2020.102150.
Xia Q, Geng F, Zhang F-F, Liu C-L, Xu P, Lu Z-Z, et al. Cyclophosphamide enhances anti-tumor effects of a fibroblast activation protein α-based DNA vaccine in tumor-bearing mice with murine breast carcinoma. Immunopharmacol Immunotoxicol. 2017;39(1):37–44. https://doi.org/10.1080/08923973.2016.1269337.
Danishmalik SN, Sin J-I. Therapeutic tumor control of HER2 DNA vaccines is achieved by an alteration of tumor cells and tumor microenvironment by gemcitabine and anti-Gr-1 Ab treatment in a HER2-expressing tumor model. DNA Cell Biol. 2017;36(9):801–11. https://doi.org/10.1089/dna.2017.3810.
Donninger H, Li C, Eaton JW, Yaddanapudi K. Cancer vaccines: promising therapeutics or an unattainable dream. Vaccines. 2021;9(6):668. https://doi.org/10.3390/vaccines9060668.
Lai X, Friedman A. Combination therapy of cancer with cancer vaccine and immune checkpoint inhibitors: a mathematical model. PLoS ONE. 2017;12(5):e0178479. https://doi.org/10.1371/journal.pone.0178479.
Finn OJ. Vaccines for cancer prevention: a practical and feasible approach to the cancer epidemic. Cancer Immunol Res. 2014;2(8):708–13. https://doi.org/10.1158/2326-6066.CIR-14-0110.
Finn OJ. The dawn of vaccines for cancer prevention. Nat Rev Immunol. 2018;18(3):183–94. https://doi.org/10.1038/nri.2017.140.
Sadeghzadeh M, Bornehdeli S, Mohahammadrezakhani H, Abolghasemi M, Poursaei E, Asadi M, et al. Dendritic cell therapy in cancer treatment; the state-of-the-art. Life Sci. 2020;254:117580. https://doi.org/10.1016/j.lfs.2020.117580.
Tacken PJ, Figdor CG. Targeted antigen delivery and activation of dendritic cells in vivo: Steps towards cost effective vaccines. Semin Immunol. 2011;23(1):12–20. https://doi.org/10.1016/j.smim.2011.01.001.
Cawood R, Hills T, Wong SL, Alamoudi AA, Beadle S, Fisher KD, et al. Recombinant viral vaccines for cancer. Trends Mol Med. 2012;18(9):564–74. https://doi.org/10.1016/j.molmed.2012.07.007.
Larocca C, Schlom J. Viral vector–based therapeutic cancer vaccines. J Cancer. 2011;17(5):359. https://doi.org/10.1097/PPO.0b013e3182325e63.
Tornesello AL, Tagliamonte M, Tornesello ML, Buonaguro FM, Buonaguro L. Nanoparticles to improve the efficacy of peptide-based cancer vaccines. Cancers [Internet]. 2020;12(4):1049.
Reche P, Flower DR, Fridkis-Hareli M, Hoshino Y. Peptide-based immunotherapeutics and vaccines 2017. J Immunol Res. 2018. https://doi.org/10.1155/2018/4568239.
Kumai T, Kobayashi H, Harabuchi Y, Celis E. Peptide vaccines in cancer—old concept revisited. Curr Opin Immunol. 2017;45:1–7. https://doi.org/10.1016/j.coi.2016.11.001.
Fioretti D, Iurescia S, Fazio VM, Rinaldi M. DNA vaccines: developing new strategies against cancer. J Biotechnol Biomed. 2010. https://doi.org/10.1155/2010/174378.
Soltani S, Farahani A, Dastranj M, Momenifar N, Mohajeri P, Emamie AD. DNA vaccine: methods and mechanisms. Adv hum biol. 2018;8(3):132. https://doi.org/10.4103/AIHB.AIHB_74_17.
Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J Hematol Oncol. 2022;15(1):1–26. https://doi.org/10.1186/s13045-022-01247-x.
Hasson SSAA, Al-Busaidi JKZ, Sallam TA. The past, current and future trends in DNA vaccine immunisations. Asian Pac J Trop Biomed. 2015;5(5):344–53. https://doi.org/10.1016/S2221-1691(15)30366-X.
Keenan BP, Jaffee EM. Whole cell vaccines—past progress and future strategies. In: Keenan BP, Jaffee EM, editors. Seminars in oncology. Amsterdam: Elsevier; 2012.
Koido S. Dendritic-tumor fusion cell-based cancer vaccines. Int J Mol Sci. 2016;17(6):828.
Kunjachan S, Ehling J, Storm G, Kiessling F, Lammers T. Noninvasive imaging of nanomedicines and nanotheranostics: principles, progress, and prospects. Chem Rev. 2015;115(19):10907–37. https://doi.org/10.1021/cr500314d.
Olusanya TOB, Haj Ahmad RR, Ibegbu DM, Smith JR, Elkordy AA. Liposomal drug delivery systems and anticancer drugs (Basel, Switzerland). Molecules. 2018. https://doi.org/10.3390/molecules23040907.
Maswadeh H. Glycosphingolipids (GSLs) from Sphingomonas paucimobilis Increase the efficacy of liposome-based nanovaccine against Acinetobacter baumannii-associated pneumonia in immunocompetent and immunocompromised mice. Molecules (Basel, Switzerland). 2022;27:7790. https://doi.org/10.3390/molecules27227790.
Sharma VK, Agrawal MK. A historical perspective of liposomes-a bio nanomaterial. Mater Today: Proc. 2021;45:2963–6. https://doi.org/10.1016/j.matpr.2020.11.952.
Butterfield LH. Cancer vaccines. BMJ. 2015. https://doi.org/10.1136/bmj.h988.
Gordon B, Gadi VK. The role of the tumor microenvironment in developing successful therapeutic and secondary prophylactic breast cancer vaccines. Vaccines. 2020;8(3):529. https://doi.org/10.3390/vaccines8030529.
Arneth B. Tumor microenvironment. Medicina. 2020;56(1):15.
Hargadon KMJC, Medicine T. Tumor microenvironmental influences on dendritic cell and T cell function: a focus on clinically relevant immunologic and metabolic checkpoints. CLIN TRANSL MED. 2020;10(1):374–411. https://doi.org/10.1002/ctm2.37.
Yan H-X, Cheng P, Wei H-Y, Shen G-B, Fu L-X, Ni J, et al. Active immunotherapy for mouse breast cancer with irradiated whole-cell vaccine expressing VEGFR2. Oncol Rep. 2013;29(4):1510–6. https://doi.org/10.3892/or.2013.2282.
Jin D, Yu X, Chen B, Li Z, Ding J, Zhao X, et al. Combined immunotherapy of breast cancer with EGF and VEGF vaccines from DNA shuffling in a mouse model. Immunotherapy. 2017;9(7):537–53. https://doi.org/10.2217/imt-2017-0004.
Zhang H, Jia E, Xia W, Lv T, Lu C, Xu Z, et al. Utilizing VEGF165b mutant as an effective immunization adjunct to augment antitumor immune response. Vaccines. 2019;37(15):2090–8. https://doi.org/10.1016/j.vaccine.2019.02.055.
Koske I, Rössler A, Pipperger L, Petersson M, Barnstorf I, Kimpel J, et al. Oncolytic virotherapy enhances the efficacy of a cancer vaccine by modulating the tumor microenvironment. Int J Cancer. 2019;145(7):1958–69. https://doi.org/10.1002/ijc.32325.
Wang B, Tian T, Kalland K-H, Ke X, Qu Y. Targeting Wnt/β-catenin signaling for cancer immunotherapy. Trends Pharmacol Sci. 2018;39(7):648–58. https://doi.org/10.1016/j.tips.2018.03.008.
Chong G, Morse M. Combining cancer vaccines with chemotherapy. Expert Opin Pharmacother. 2005;6(16):2813–20. https://doi.org/10.1080/17460441.2020.1811673.
Beyranvand Nejad E, Welters MJ, Arens R, van der Burg SH. The importance of correctly timing cancer immunotherapy. Expert Opin Biol Ther. 2017;17(1):87–103. https://doi.org/10.1080/14712598.2017.1256388.
Schlom J, Arlen PM, Gulley JL. Cancer vaccines: moving beyond current paradigms. Clin Cancer Res. 2007;13(13):3776–82. https://doi.org/10.1158/1078-0432.CCR-07-0588.
Fridman WH, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. https://doi.org/10.1038/nrc3245.
Vacchelli E, Aranda F, Eggermont A, Galon J, Sautès-Fridman C, Cremer I, et al. Trial Watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2014;3(3):e27878. https://doi.org/10.4161/onci.27878.
Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, et al. CD4+ CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34(2):336–44. https://doi.org/10.1002/eji.200324181.
Banissi C, Ghiringhelli F, Chen L, Carpentier AF. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother. 2009;58(10):1627–34. https://doi.org/10.1007/s00262-009-0671-1.
Litterman AJ, Dudek AZ, Largaespada DA. Alkylating chemotherapy may exert a uniquely deleterious effect upon neo-antigen-targeting anticancer vaccination. Oncoimmunology. 2013;2(10):e26294. https://doi.org/10.4161/onci.26294.
Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Investig J. 2006;12(3):878–87. https://doi.org/10.1158/1078-0432.CCR-05-2013.
Chemotherapy Followed By Vaccine Therapy in Treating Patients With Extensive-Stage Small Cell Lung Cancer [Available from: https://clinicaltrials.gov/ct2/show/record/NCT00049218?term=p53+Cancer+Vaccine+with+Chemotherapy&cond=Extensive+Stage+Small+Cell+Lung+Cancer&draw=2&rank=1.
Wu X, Feng Q-M, Wang Y, Shi J, Ge H-L, Di W. The immunologic aspects in advanced ovarian cancer patients treated with paclitaxel and carboplatin chemotherapy. Cancer Immunol Immunother. 2010;59(2):279–91. https://doi.org/10.1007/s00262-009-0749-9.
Murahashi M, Hijikata Y, Yamada K, Tanaka Y, Kishimoto J, Inoue H, et al. Phase I clinical trial of a five-peptide cancer vaccine combined with cyclophosphamide in advanced solid tumors. Clin Immunol. 2016;166:48–58. https://doi.org/10.1016/j.clim.2016.03.015.
Farsaci B, Higgins JP, Hodge JW. Consequence of dose scheduling of sunitinib on host immune response elements and vaccine combination therapy. Int J Cancer. 2012;130(8):1948–59. https://doi.org/10.1002/ijc.26219.
Ramakrishnan R, Antonia S, Gabrilovich DI. Combined modality immunotherapy and chemotherapy: a new perspective. Cancer Immunol Immunother. 2008;57(10):1523–9. https://doi.org/10.1007/s00262-008-0531-4.
Chemo-immunotherapy (Gemcitabine, Interferon-alpha 2b and p53 SLP) in Patients With Platinum-resistant Ovarian Cancer (CHIP) [Available from: https://clinicaltrials.gov/ct2/show/NCT01639885?term=gemcitabine%2C+Pegintron+and+p53+SLP+vaccine&cond=Platinum-resistant+Ovarian+Cancer&draw=2&rank=1.
Yang F, Shi K, Hao Y, Jia Y, Liu Q, Chen Y, et al. Cyclophosphamide loaded thermo-responsive hydrogel system synergize with a hydrogel cancer vaccine to amplify cancer immunotherapy in a prime-boost manner. Bioact Mater. 2021;6(10):3036–48. https://doi.org/10.1016/j.bioactmat.2021.03.003.
Berinstein NL, Karkada M, Oza AM, Odunsi K, Villella JA, Nemunaitis JJ, et al. Survivin-targeted immunotherapy drives robust polyfunctional T cell generation and differentiation in advanced ovarian cancer patients. Oncoimmunology. 2015;4(8):e1026529. https://doi.org/10.1080/2162402X.2015.1026529.
Gibney GT, Kudchadkar RR, DeConti RC, Thebeau MS, Czupryn MP, Tetteh L, et al. Safety, Correlative markers, and clinical results of adjuvant Nivolumab in combination with vaccine in resected high-risk metastatic Melanomaadjuvant anti–PD-1 therapy in resected melanoma patients. Clin Cancer Res. 2015;21(4):712–20. https://doi.org/10.1158/1078-0432.CCR-14-2468.
Tagliamonte M, Petrizzo A, Napolitano M, Luciano A, Arra C, Maiolino P, et al. Novel metronomic chemotherapy and cancer vaccine combinatorial strategy for hepatocellular carcinoma in a mouse model. Cancer Immunol Immunother. 2015;64(10):1305–14. https://doi.org/10.1007/s00262-015-1698-0.
van der Sluis TC, van Duikeren S, Huppelschoten S, Jordanova ES, Beyranvand Nejad E, Sloots A, et al. Vaccine-induced tumor necrosis factor-producing T cells synergize with cisplatin to promote tumor cell deathsynergy between T cell-produced cytokines and cisplatin. Clin Cancer Res. 2015;21(4):781–94. https://doi.org/10.1158/1078-0432.CCR-14-2142.
Phase II Trial of Combination Immunotherapy With NeuVax and Trastuzumab in High-risk HER2+ Breast Cancer Patients (HER3+) [Available from: https://clinicaltrials.gov/ct2/show/study/NCT02297698?term=vaccination+in+combination+with+chemotherapy&recrs=e&cond=Cancer&phase=123&draw=2&rank=7.
Campf J, Clifton GT, Hale DF, Vreeland TJ, Hickerson A, Holmes JP, et al. Immunologic responses in triple-negative breast cancer patients in a randomized phase IIb trial of nelipepimut-S plus trastuzumab versus trastuzumab alone to prevent recurrence. Am Soc Clin Oncol. 2019. https://doi.org/10.1200/JCO.2019.37.15_suppl.556.
Combination Immunotherapy With Herceptin and the HER2 Vaccine NeuVax [Available from: https://clinicaltrials.gov/ct2/show/study/NCT01570036?term=vaccination+in+combination+with+chemotherapy&recrs=e&cond=Cancer&phase=123&draw=2&rank=8.
Clifton GT, Hale D, Vreeland TJ, Hickerson AT, Litton JK, Alatrash G, et al. Results of a randomized Phase IIb trial of Nelipepimut-S+ Trastuzumab versus Trastuzumab to prevent recurrences in patients with high-risk HER2 low-expressing breast cancer. Clin Cancer Res. 2020;26(11):2515–23. https://doi.org/10.1158/1078-0432.CCR-19-2741.
Platin-based Chemotherapeutics to Enhance Dendritic Cell Vaccine Efficacy in Melanoma Patients [Available from: https://clinicaltrials.gov/ct2/show/record/NCT02285413?term=vaccination+in+combination+with+chemotherapy&recrs=e&cond=Cancer&phase=123&draw=2&rank=20.
Boudewijns S, Bloemendal M, de Haas N, Westdorp H, Bol KF, Schreibelt G, et al. Autologous monocyte-derived DC vaccination combined with cisplatin in stage III and IV melanoma patients: a prospective, randomized phase 2 trial. Cancer Immunol Immunother. 2020;69(3):477–88. https://doi.org/10.1007/s00262-019-02466-x.
Docetaxel Alone or in Combination With Vaccine to Treat Breast Cancer [Available from: https://clinicaltrials.gov/ct2/show/record/NCT00179309?term=vaccination+in+combination+with+chemotherapy&recrs=e&cond=Cancer&phase=123&draw=2&rank=10.
Heery CR, Ibrahim NK, Arlen PM, Mohebtash M, Murray JL, Koenig K, et al. Docetaxel alone or in combination with a therapeutic cancer vaccine (PANVAC) in patients with metastatic breast cancer: a randomized clinical trial. JAMA Oncol. 2015;1(8):1087–95. https://doi.org/10.1001/jamaoncol.2015.2736.
Vaccine Therapy With or Without Cyclophosphamide in Treating Patients Undergoing Chemotherapy and Radiation Therapy for Stage I or Stage II Pancreatic Cancer That Can Be Removed by Surgery [Available from: https://clinicaltrials.gov/ct2/show/results/NCT00727441?term=vaccination+in+combination+with+chemotherapy&recrs=e&cond=Cancer&phase=123&draw=2&rank=6.
Bloch O. Immunotherapy for malignant gliomas Current Understanding Treatment of Gliomas. Cham: Springer; 2015. p. 143–58.
Vaccine Therapy in Treating Patients With Newly Diagnosed Glioblastoma Multiforme (ACTIVATe) [Available from: https://clinicaltrials.gov/ct2/show/record/NCT00643097?term=vaccination+in+combination+with+chemotherapy&recrs=e&cond=Cancer&phase=123&draw=2&rank=17.
Vogelzang NJ, Beer TM, Gerritsen W, Oudard S, Wiechno P, Kukielka-Budny B, et al. Efficacy and Safety of autologous dendritic cell-based immunotherapy, docetaxel, and prednisone vs placebo in patients with metastatic castration-resistant prostate cancer: the VIABLE phase 3 randomized clinical trial. JAMA Oncol. 2022;8(4):546–52. https://doi.org/10.1001/jamaoncol.2021.7298.
Phase III Study of DCVAC/PCa Added to Standard Chemotherapy for Men With Metastatic Castration Resistant Prostate Cancer [Available from: https://clinicaltrials.gov/ct2/show/record/NCT02111577?term=combination+of+chemotherapy+and+cancer+vaccine+therapy&recrs=e&cond=Cancer&phase=23&draw=2&rank=5.
Vaccine Therapy, Cyclophosphamide, and Cetuximab in Treating Patients With Metastatic or Locally Advanced Pancreatic Cancer [Available from: https://clinicaltrials.gov/ct2/show/NCT00305760?term=combining+chemotherapy+and+cancer+vaccine+therapy&recrs=e&cond=Cancer&phase=123&draw=2&rank=9.
A Trial of Perioperative CV301 Vaccination in Combination With Nivolumab and Systemic Chemotherapy for Metastatic CRC [Available from: https://clinicaltrials.gov/ct2/show/record/NCT03547999?term=vaccination+in+combination+with+chemotherapy&recrs=adf&cond=Cancer&phase=123&draw=2&rank=1&view=record.
Autologous Dendritic Cell Vaccination in Mesothelioma (MESODEC) [Available from: https://clinicaltrials.gov/ct2/show/record/NCT02649829?term=vaccination+in+combination+with+chemotherapy&recrs=adf&cond=Cancer&phase=123&draw=2&rank=5&view=record.
Adjuvant Dendritic Cell-immunotherapy Plus Temozolomide in Glioblastoma Patients (ADDIR-GLIO) [Available from: https://clinicaltrials.gov/ct2/show/record/NCT02649582?term=vaccination+in+combination+with+chemotherapy&recrs=adf&cond=Cancer&phase=123&draw=2&rank=22&view=record.
Concurrent WOKVAC Vaccination, Chemotherapy, and HER2-Targeted Monoclonal Antibody Therapy Before Surgery for the Treatment of Patients With Breast Cancer [Available from: https://clinicaltrials.gov/ct2/show/study/NCT04329065?term=vaccination+in+combination+with+chemotherapy&recrs=adf&cond=Cancer&phase=123&draw=2&rank=2.
Vaccination of Triple Negative Breast Cancer Patients [Available from: https://clinicaltrials.gov/ct2/show/study/NCT02229084?term=vaccination+in+combination+with+chemotherapy&recrs=adf&cond=Cancer&phase=123&draw=2&rank=4.
GVAX Pancreas Vaccine (With CY) in Combination With Nivolumab and SBRT for Patients With Borderline Resectable Pancreatic Cancer [Available from: https://clinicaltrials.gov/ct2/show/record/NCT03161379?term=vaccination+in+combination+with+chemotherapy&recrs=adf&cond=Cancer&phase=123&draw=2&rank=9.
Therapeutic Cancer Vaccine (AST-301, pNGVL3-hICD) in Patients With Breast Cancer [Available from: https://clinicaltrials.gov/ct2/show/study/NCT05163223?term=vaccination+in+combination+with+chemotherapy&recrs=adf&cond=Cancer&phase=123&draw=2&rank=12.
HPV-16 Vaccination and Pembrolizumab Plus Cisplatin for “Intermediate Risk” HPV-16-associated Head and Neck Squamous Cell Carcinoma [Available from: https://clinicaltrials.gov/ct2/show/record/NCT04369937?term=vaccination+in+combination+with+chemotherapy&recrs=adf&cond=Cancer&phase=123&draw=2&rank=21.
Tagliamonte M, Petrizzo A, Mauriello A, Tornesello ML, Buonaguro FM, Buonaguro L. Potentiating cancer vaccine efficacy in liver cancer. Oncoimmunology. 2018;7(10):e1488564. https://doi.org/10.1080/2162402X.2018.1488564.
Luo Q, Zhang L, Luo C, Jiang M. Emerging strategies in cancer therapy combining chemotherapy with immunotherapy. Cancer Lett. 2019;454:191–203. https://doi.org/10.1016/j.canlet.2019.04.017.
Cancer Vaccines: Types, Schedule and Limitations [Available from: https://onco.com/blog/cancer-vaccines-types-schedule-and-limitations/#:~:text=People%20with%20a%20weak%20immune,hamper%20its%20ability%20to%20respond.
Conforti A, Salvatori E, Lione L, Compagnone M, Pinto E, Shorrock C, et al. Linear DNA amplicons as a novel cancer vaccine strategy. J Exp Clin Cancer Res. 2022;41(1):1–13. https://doi.org/10.1186/s13046-022-02402-5.
Tsiatas M, Mountzios G, Curigliano G. Future perspectives in cancer immunotherapy. Ann Transl Med. 2016;4(14):273. https://doi.org/10.21037/atm.2016.07.14.
Acknowledgements
The authors are grateful to Prof. Gaurang B. Shah, Department of Pharmacology, L. M. College of Pharmacy, Ahmedabad, Gujarat, India for kind support and guidance in manuscript preparation. The authors also extend their appreciation to L. M. College of Pharmacy, Ahmedabad, India for providing continuous library resources support throughout literature survey and data collection.
Funding
The authors declare that no funds, grants, or other support have been received during or for the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
NK, HP, AP, AS, YS—Manuscript first draft preparation and subsequent editing, Literature and data survey, Figures and diagram conception and designing. MRC—Review topic conception, Design of content and skeleton, Manuscript draft review and editing; Figures and Tables conception, Designing and Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest to report.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kothari, N., Postwala, H., Pandya, A. et al. Establishing the applicability of cancer vaccines in combination with chemotherapeutic entities: current aspect and achievable prospects. Med Oncol 40, 135 (2023). https://doi.org/10.1007/s12032-023-02003-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02003-y