Abstract
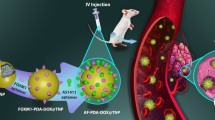
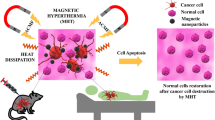
Cancer is a major cause of death worldwide. Cancer-resistant to chemo or radiotherapy treatment is a challenge that could be overcome by a nanotechnology approach. Providing a theranostic nano-platform for different cancer treatment strategies could be revolutionary. Here we introduce a multifunctional theranostic nanostructure which has the capacity for improving cancer diagnosis and treatment through better chemo and radiotherapy and current x-ray imaging systems through co-encapsulation of a small gold cluster and anticancer drug doxorubicin. 2 nm gold clusters represent good heating under radio frequency electric field (RF-EF) exposure and have been used for in vitro hyperthermia treatment of cancerous cells. Liposomal doxorubicin (169 ± 19.8 nm) with gold clusters encapsulation efficiency of 13.2 ± 3.0% and doxorubicin encapsulation efficiency of 64.7 ± 0.7% were prepared and studied as a theranostic agent with a high potential in different cancer treatment modalities. Exposure to a radiofrequency electric field on prepared formulation caused 20.2 ± 2.1% drug release and twice decreasing of IC50 on colorectal carcinoma cells. X-ray attenuation efficiency of the liposomal gold cluster was better than commercial iohexol and free gold clusters in different concentrations. Finally, treatment of gold clusters on cancerous cells results in a significant decrease in the viability of irradiated cells to cobalt-60 beam. Based on these experiments, we concluded that the conventional liposomal formulation of doxorubicin that has been co-encapsulated with small gold clusters could be a suitable theranostic nanostructure for cancer treatment and merits further investigation.








Similar content being viewed by others
References
DeAngelis LM, et al. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93–10. J Clin Oncol. 2002;20(24):4643–8.
Sriraman SK, Aryasomayajula B, Torchilin VP. Barriers to drug delivery in solid tumors. Tissue Barriers. 2014;2(3): e29528.
Fatemi F, et al. Construction of genetically engineered M13K07 helper phage for simultaneous phage display of gold binding peptide 1 and nuclear matrix protein 22 ScFv antibody. Colloids Surf B. 2017;159:770–80.
Koosha F, et al. Mesoporous silica coated gold nanorods: a multifunctional theranostic platform for radiotherapy and X-ray imaging. J Porous Mater. 2021;28(6):1961–8.
Neshastehriz A, et al. In-vitro investigation of green synthesized gold nanoparticle's role in combined photodynamic and radiation therapy of cancerous cells. Adv Nat Sci. 2020;11(4).
Kamalabadi M, et al. Folate functionalized gold-coated magnetic nanoparticles effect in combined electroporation and radiation treatment of HPV-positive oropharyngeal cancer. Med Oncol. 2022;39(12).
Baijal G, et al. Comparative study of one pot synthesis of PEGylated gold and silver nanoparticles for imaging and radiosensitization of oral cancers. Radiat Phys Chem. 2022;194: 109990.
Lin M-H, et al. Comparison of organic and inorganic germanium compounds in cellular radiosensitivity and preparation of germanium nanoparticles as a radiosensitizer. Int J Radiat Biol. 2009;85(3):214–26.
Porcel E, et al. Platinum nanoparticles: a promising material for future cancer therapy? Nanotechnology. 2010;21(8): 085103.
Shirkhanloo H, et al. Novel semisolid design based on bismuth oxide (Bi2O3) nanoparticles for radiation protection. Nanomed Res J. 2017;2(4):230–8.
Chithrani DB, et al. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat Res. 2010;173(6):719–28.
Zhang X-D, et al. In vivo renal clearance, biodistribution, toxicity of gold nanoclusters. Biomaterials. 2012;33(18):4628–38.
Khlebtsov N, Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev. 2011;40(3):1647–71.
Hirn S, et al. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur J Pharm Biopharm. 2011;77(3):407–16.
Drummond DC, et al. Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. J Pharm Sci. 2008;97(11):4696–740.
Drummond DC, et al. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev. 1999;51(4):691–744.
Halm U, et al. A phase II study of pegylated liposomal doxorubicin for treatment of advanced hepatocellular carcinoma. Ann Oncol. 2000;11(1):113–4.
Chidiac T, et al. Phase II trial of liposomal doxorubicin (Doxil®) in advanced soft tissue sarcomas. Invest New Drugs. 2000;18(3):253–9.
Laginha KM, et al. Determination of doxorubicin levels in whole tumor and tumor nuclei in murine breast cancer tumors. Clin Cancer Res. 2005;11(19):6944–9.
Tseng Y-L, Liu J-J, Hong R-L. Translocation of liposomes into cancer cells by cell-penetrating peptides penetratin and tat: a kinetic and efficacy study. Mol Pharmacol. 2002;62(4):864–72.
Ng K-Y, et al. The effects of polyethyleneglycol (PEG)-derived lipid on the activity of target-sensitive immunoliposome. Int J Pharm. 2000;193(2):157–66.
Parr MJ, et al. Accumulation of liposomal lipid and encapsulated doxorubicin in murine Lewis lung carcinoma: the lack of beneficial effects by coating liposomes with poly (ethylene glycol). J Pharmacol Exp Ther. 1997;280(3):1319–27.
Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48.
Biabangard A, et al. Study of FA12 peptide-modified PEGylated liposomal doxorubicin (PLD) as an effective ligand to target Muc1 in mice bearing C26 colon carcinoma: in silico, in vitro, and in vivo study. Expert Opin Drug Deliv. 2022;19(12):1710–24.
Bibi S, et al. Trigger release liposome systems: local and remote controlled delivery? J Microencapsul. 2012;29(3):262–76.
Amini SM, Kharrazi S, Jaafari MR. Radio frequency hyperthermia of cancerous cells with gold nanoclusters: an in vitro investigation. Gold Bull. 2017;50(1):43–50.
Amin M, Badiee A, Jaafari MR. Improvement of pharmacokinetic and antitumor activity of PEGylated liposomal doxorubicin by targeting with N-methylated cyclic RGD peptide in mice bearing C-26 colon carcinomas. Int J Pharm. 2013;458(2):324–33.
Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem. 1959;234:466–8.
Bolotin EM, et al. Ammonium sulfate gradients for efficient and stable remote loading of amphipathic weak bases into liposomes and ligandoliposomes. J Liposome Res. 1994;4(1):455–79.
Haran G, et al. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim Biophys Acta. 1993;1151(2):201–15.
Huang Z, Jaafari MR, Szoka FC Jr. Disterolphospholipids: nonexchangeable lipids and their application to liposomal drug delivery. Angew Chem. 2009;121(23):4210–3.
Siegel MJ, et al. Radiation dose and image quality in pediatric CT: effect of technical factors and phantom size and shape 1. Radiology. 2004;233(2):515–22.
Pradhan P, et al. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Control Release. 2010;142(1):108–21.
Lorenzato C, et al. MRI contrast variation of thermosensitive magnetoliposomes triggered by focused ultrasound: a tool for image-guided local drug delivery. Contrast Media Mol Imaging. 2013;8(2):185–92.
Zarchi AAK, et al. Synthesis and characterisation of liposomal doxorubicin with loaded gold nanoparticles. IET Nanobiotechnol. 2018;12:846–9.
Mathiyazhakan M, et al. Non-invasive controlled release from gold nanoparticle integrated photo-responsive liposomes through pulse laser induced microbubble cavitation. Colloids Surf B. 2015;126:569–74.
Xia Y, et al. Construction of thermal-and light-responsive liposomes noncovalently decorated with gold nanoparticles. RSC Adv. 2014;4(84):44568–74.
Demir B, et al. Gold nanoparticle loaded phytosomal systems: synthesis, characterization and in vitro investigations. RSC Adv. 2014;4(65):34687–95.
Freeny PC, et al. Colorectal carcinoma evaluation with CT: preoperative staging and detection of postoperative recurrence. Radiology. 1986;158(2):347–53.
Rifkin MD, Ehrlich S, Marks G. Staging of rectal carcinoma: prospective comparison of endorectal US and CT. Radiology. 1989;170(2):319–22.
Lusic H, Grinstaff MW. X-ray-computed tomography contrast agents. Chem Rev. 2012;113(3):1641–66.
Hainfeld, J., et al. Gold nanoparticles: a new X-ray contrast agent. Br J Radiol 2014.
Rabin O, et al. An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nat Mater. 2006;5(2):118–22.
Kandanapitiye MS, et al. Synthesis, characterization, and X-ray attenuation properties of ultrasmall BiOI nanoparticles: toward renal clearable particulate CT contrast agents. Inorg Chem. 2014;53(19):10189–94.
Liu Z, et al. Long-circulating Er 3+-doped Yb 2 O 3 up-conversion nanoparticle as an in vivo X-Ray CT imaging contrast agent. Biomaterials. 2012;33(28):6748–57.
Liu Y, et al. A high-performance ytterbium-based nanoparticulate contrast agent for in vivo X-ray computed tomography imaging. Angew Chem Int Ed. 2012;51(6):1437–42.
Lechtman E, et al. Implications on clinical scenario of gold nanoparticle radiosensitization in regards to photon energy, nanoparticle size, concentration and location. Phys Med Biol. 2011;56(15):4631.
Khoshgard K, et al. Radiosensitization effect of folate-conjugated gold nanoparticles on HeLa cancer cells under orthovoltage superficial radiotherapy techniques. Phys Med Biol. 2014;59(9):2249.
Hainfeld JF, et al. Radiotherapy enhancement with gold nanoparticles. J Pharm Pharmacol. 2008;60(8):977–85.
Leung MK, et al. Irradiation of gold nanoparticles by x-rays: Monte Carlo simulation of dose enhancements and the spatial properties of the secondary electrons production. Med Phys. 2011;38(2):624–31.
Hill R, et al. Advances in kilovoltage X-ray beam dosimetry. Phys Med Biol. 2014;59(6):R183.
Goodman CM, et al. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem. 2004;15(4):897–900.
Marquis BJ, et al. Analytical methods to assess nanoparticle toxicity. Analyst. 2009;134(3):425–39.
Niidome T, et al. PEG-modified gold nanorods with a stealth character for in vivo applications. J Control Release. 2006;114(3):343–7.
Mathew A, Pradeep T. Noble metal clusters: applications in energy, environment, and biology. Part Part Syst Charact. 2014;31(10):1017–53.
Mayer L, Bally M, Cullis P. Uptake of adriamycin into large unilamellar vesicles in response to a pH gradient. Biochim Biophys Acta. 1986;857(1):123–6.
Hanson GW, Monreal R, Apell SP. Electromagnetic absorption mechanisms in metal nanospheres: Bulk and surface effects in radiofrequency-terahertz heating of nanoparticles. J Appl Phys. 2011;109(12): 124306.
Acknowledgements
This work has been funded by the Tehran University of Medical Sciences (93-01-87-25211). The authors are grateful to Dr. Hossein Ghadiri for providing the scanning holder for computed tomography experiments.
Funding
Funding was provided by Tehran University of Medical Sciences and Health Services.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amini, S.M., Rezayat, S.M., Dinarvand, R. et al. Gold cluster encapsulated liposomes: theranostic agent with stimulus triggered release capability. Med Oncol 40, 126 (2023). https://doi.org/10.1007/s12032-023-01991-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-01991-1