Abstract
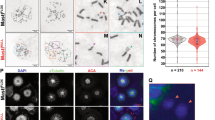
PTEN, dual phosphatase tumor suppressor protein, is found to be frequently mutated in various cancers. Post-translational modification of PTEN is important for its sub-cellular localization and catalytic functions. But how these modifications affect cytological damage and aneuploidy is not studied in detail. We focus on the role of phosphatase activity along with C-terminal phosphorylation of PTEN in perspective of cytological damage like micronucleus, nuclear bud, and nuclear bridge formation. Our data suggest that wild-type PTEN, but not phospho-mutant PTEN significantly reduces cytological damage in PTEN null PC3 cells. In case of phosphatase-dead PTEN, cytological damage markers are increased during 24 h recovery after DNA damage. When we use phosphorylation and phosphatase-dead dual mutant PTEN, the extent of different cytological DNA damage parameters are similar to phosphatase-dead PTEN. We also find that both of those activities are essential for maintaining chromosome numbers. PTEN null cells exhibit significantly aberrant γ-tubulin pole formation during metaphase. Interestingly, we observed that p-PTEN localized to spindle poles along with PLK1 and Aurora Kinase A. Further depletion of phosphorylation and phosphatase activity of PTEN increases the expression of p-Aurora Kinase A (T288) and p-PLK1 (T210), compared to cells expressing wild-type PTEN. Again, wild-type PTEN but not phosphorylation-dead mutant is able to physically interact with PLK1 and Aurora Kinase A. Thus, our study suggests that the phosphorylation-dependent interaction of PTEN with PLK1 and Aurora Kinase A causes dephosphorylation of those mitotic kinases and by lowering their hyperphosphorylation status, PTEN prevents aberrant chromosome segregation in metaphase.





Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275(5308):1943–7. https://doi.org/10.1126/science.275.5308.1943. (PMID: 9072974).
Eng C. PTEN: one gene, many syndromes. Hum Mutat. 2003;22(3):183–98. https://doi.org/10.1002/humu.10257. (PMID: 12938083).
Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15(4):356–62. https://doi.org/10.1038/ng0497-356. (PMID: 9090379).
Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA, Wigler MH, Downes CP, Tonks NK. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proc Natl Acad Sci USA. 1998;95(23):13513–8. https://doi.org/10.1073/pnas.95.23.13513.PMID:9811831;PMCID:PMC24850.
Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95(1):29–39. https://doi.org/10.1016/s0092-8674(00)81780-8. (PMID: 9778245).
Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, Yin Y. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128(1):157–70. https://doi.org/10.1016/j.cell.2006.11.042. (PMID: 17218262).
Bassi C, Ho J, Srikumar T, Dowling RJ, Gorrini C, Miller SJ, Mak TW, Neel BG, Raught B, Stambolic V. Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science. 2013;341(6144):395–9. https://doi.org/10.1126/science.1236188.Erratum.In:Science.2013Sep6;341(6150):1064.PMID:23888040;PMCID:PMC5087104.
Hou SQ, Ouyang M, Brandmaier A, Hao H, Shen WH. PTEN in the maintenance of genome integrity: From DNA replication to chromosome segregation. BioEssays. 2017;39(10):1700082. https://doi.org/10.1002/bies.201700082.
Brandmaier A, Hou SQ, Shen WH. Cell cycle control by PTEN. J Mol Biol. 2017;429(15):2265–77. https://doi.org/10.1016/j.jmb.2017.06.004.
Mansour WY, Tennstedt P, Volquardsen J, Oing C, Kluth M, Hube-Magg C, Borgmann K, Simon R, Petersen C, Dikomey E, Rothkamm K. Loss of PTEN-assisted G2/M checkpoint impedes homologous recombination repair and enhances radio-curability and PARP inhibitor treatment response in prostate cancer. Sci Rep. 2018;8(1):3947. https://doi.org/10.1038/s41598-018-22289-7.PMID:29500400;PMCID:PMC5834544.
He J, Kang X, Yin Y, Chao KS, Shen WH. PTEN regulates DNA replication progression and stalled fork recovery. Nat Commun. 2015;9(6):7620. https://doi.org/10.1038/ncomms8620.PMID:26158445;PMCID:PMC4499867.
Sun Z, Lu J, Wu M, Li M, Bai L, Shi Z, Hao L, Wu Y. Deficiency of PTEN leads to aberrant chromosome segregation through downregulation of MAD2. Mol Med Rep. 2019;20(5):4235–43. https://doi.org/10.3892/mmr.2019.10668.
Yuen KW, Montpetit B, Hieter P. The kinetochore and cancer: what’s the connection? Curr Opin Cell Biol. 2005;17(6):576–82. https://doi.org/10.1016/j.ceb.2005.09.012. (Epub 2005 Oct 17 PMID: 16233975).
Puc J, Keniry M, Li HS, Pandita TK, Choudhury AD, Memeo L, Mansukhani M, Murty VV, Gaciong Z, Meek SE, Piwnica-Worms H, Hibshoosh H, Parsons R. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell. 2005;7(2):193–204. https://doi.org/10.1016/j.ccr.2005.01.009. (PMID: 15710331).
Sun Z, Huang C, He J, Lamb KL, Kang X, Gu T, Shen WH, Yin Y. PTEN C-terminal deletion causes genomic instability and tumor development. Cell Rep. 2014;6(5):844–54. https://doi.org/10.1016/j.celrep.2014.01.030.
Vazquez F, Grossman SR, Takahashi Y, Rokas MV, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J Biol Chem. 2001;276(52):48627–30. https://doi.org/10.1074/jbc.C100556200. (Epub 2001 Nov 13 PMID: 11707428).
Lang V, Aillet F, Da Silva-Ferrada E, Xolalpa W, Zabaleta L, Rivas C, Rodriguez MS. Analysis of PTEN ubiquitylation and SUMOylation using molecular traps. Methods. 2015;77–78:112–8. https://doi.org/10.1016/j.ymeth.2014.09.001. (Epub 2014 Sep 16 PMID: 25224693).
Meng Z, Jia LF, Gan YH. PTEN activation through K163 acetylation by inhibiting HDAC6 contributes to tumour inhibition. Oncogene. 2016;35(18):2333–44. https://doi.org/10.1038/onc.2015.293. (Epub 2015 Aug 17 PMID: 26279303).
Zhang Y, Park J, Han SJ, Yang SY, Yoon HJ, Park I, Woo HA, Lee SR. Redox regulation of tumor suppressor PTEN in cell signaling. Redox Biol. 2020;34:101553. https://doi.org/10.1016/j.redox.2020.101553.
Choi BH, Pagano M, Dai W. Plk1 protein phosphorylates phosphatase and tensin homolog (PTEN) and regulates its mitotic activity during the cell cycle. J Biol Chem. 2014;289(20):14066–74. https://doi.org/10.1074/jbc.M114.558155.
Al-Khouri AM, Ma Y, Togo SH, Williams S, Mustelin T. Cooperative phosphorylation of the tumor suppressor phosphatase and tensin homologue (PTEN) by casein kinases and glycogen synthase kinase 3beta. J Biol Chem. 2005;280(42):35195–202. https://doi.org/10.1074/jbc.M503045200. (Epub 2005 Aug 17 PMID: 16107342).
Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000;20(14):5010–8. https://doi.org/10.1128/mcb.20.14.5010-5018.2000.PMID:10866658;PMCID:PMC85951.
Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus: implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276(2):993–8. https://doi.org/10.1074/jbc.M009134200. (PMID: 11035045).
Ginn-Pease ME, Eng C. Increased nuclear phosphatase and tensin homologue deleted on chromosome 10 is associated with G0–G1 in MCF-7 cells. Cancer Res. 2003;63(2):282–6 (PMID: 12543774).
Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci USA. 1999;96(11):6199–204. https://doi.org/10.1073/pnas.96.11.6199.PMID:10339565;PMCID:PMC26859.
Ma J, Benitez JA, Li J, Miki S, Ponte de Albuquerque C, Galatro T, Orellana L, Zanca C, Reed R, Boyer A, Koga T, Varki NM, Fenton TR, Nagahashi Marie SK, Lindahl E, Gahman TC, Shiau AK, Zhou H, DeGroot J, Sulman EP, Cavenee WK, Kolodner RD, Chen CC, Furnari FB. Inhibition of nuclear PTEN tyrosine phosphorylation enhances glioma radiation sensitivity through attenuated DNA repair. Cancer Cell. 2019;35(3):504–18.e7. doi: https://doi.org/10.1016/j.ccell.2019.01.020. Epub 2019 Feb 28. Erratum in: Cancer Cell. 2019 May 13;35(5):816. Erratum in: Cancer Cell. 2019 Dec 9;36(6):690–91. PMID: 30827889; PMCID: PMC6424615.
Misra S, Chowdhury SG, Ghosh G, Mukherjee A, Karmakar P. Both phosphorylation and phosphatase activity of PTEN are required to prevent replication fork progression during stress by inducing heterochromatin. Mutation Res/Fundamental Mol Mech Mutagen. 2022;825:111800. https://doi.org/10.1016/j.mrfmmm.2022.111800.
Barr FA, Silljé HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5(6):429–40. https://doi.org/10.1038/nrm1401. (PMID: 15173822).
Strebhardt K. Multifaceted polo-like kinases: drug targets and antitargets for cancer therapy. Nat Rev Drug Discov. 2010;9(8):643–60. https://doi.org/10.1038/nrd3184. (PMID: 20671765).
Liu X, Erikson RL. Polo-like kinase (Plk)1 depletion induces apoptosis in cancer cells. Proc Natl Acad Sci USA. 2003;100(10):5789–94. https://doi.org/10.1073/pnas.1031523100.
Smits VA, Klompmaker R, Arnaud L, Rijksen G, Nigg EA, Medema RH. Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat Cell Biol. 2000;2(9):672–6. https://doi.org/10.1038/35023629. (PMID: 10980711).
Liu XS, Li H, Song B, Liu X. Polo-like kinase 1 phosphorylation of G2 and S-phase-expressed 1 protein is essential for p53 inactivation during G2 checkpoint recovery. EMBO Rep. 2010;11(8):626–32. https://doi.org/10.1038/embor.2010.90.
Bassermann F, Frescas D, Guardavaccaro D, Busino L, Peschiaroli A, Pagano M. The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell. 2008;134(2):256–67. https://doi.org/10.1016/j.cell.2008.05.043.PMID:18662541;PMCID:PMC2591934.
van Vugt MA, Brás A, Medema RH. Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol Cell. 2004;15(5):799–811. https://doi.org/10.1016/j.molcel.2004.07.015. (PMID: 15350223).
Meraldi P, Honda R, Nigg EA. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53-/- cells. EMBO J. 2002;21(4):483–92. https://doi.org/10.1093/emboj/21.4.483.PMID:11847097;PMCID:PMC125866.
Fu J, Bian M, Jiang Q, Zhang C. Roles of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res. 2007;5(1):1–10. https://doi.org/10.1158/1541-7786.MCR-06-0208. (PMID: 17259342).
Joukov V, De Nicolo A. Aurora-PLK1 cascades as key signaling modules in the regulation of mitosis. Sci Signal. 2018;11(543):4195. https://doi.org/10.1126/scisignal.aar4195. (PMID: 30108183).
Sen S, Zhou H, Zhang RD, Yoon DS, Vakar-Lopez F, Ito S, Jiang F, Johnston D, Grossman HB, Ruifrok AC, Katz RL, Brinkley W, Czerniak B. Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J Natl Cancer Inst. 2002;94(17):1320–9. https://doi.org/10.1093/jnci/94.17.1320. (PMID: 12208897).
Staff S, Isola J, Jumppanen M, Tanner M. Aurora-A gene is frequently amplified in basal-like breast cancer. Oncol Rep. 2010;23(2):307–12 (PMID: 20043089).
Yang SB, Zhou XB, Zhu HX, Quan LP, Bai JF, He J, Gao YN, Cheng SJ, Xu NZ. Amplification and overexpression of Aurora-A in esophageal squamous cell carcinoma. Oncol Rep. 2007;17(5):1083–8 (PMID: 17390048).
Mukherjee A, Misra S, Howlett NG, Karmakar P. Multinucleation regulated by the Akt/PTEN signaling pathway is a survival strategy for HepG2 cells. Mutat Res. 2013;755(2):135–40. https://doi.org/10.1016/j.mrgentox.2013.06.009. (Epub 2013 Jun 21 PMID: 23796964).
Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3(1):51–62. https://doi.org/10.1016/s1535-6108(02)00235-0. (PMID: 12559175).
Fenech M. The in vitro micronucleus technique. Mutat Res. 2000;455(1–2):81–95. https://doi.org/10.1016/s0027-5107(00)00065-8. (PMID: 11113469).
Zorba A, Buosi V, Kutter S, Kern N, Pontiggia F, Cho YJ, Kern D. Molecular mechanism of Aurora A kinase autophosphorylation and its allosteric activation by TPX2. Elife. 2014;3:e02667. https://doi.org/10.7554/eLife.02667.
Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer. 2010;10(12):825–41. https://doi.org/10.1038/nrc2964. (Epub 2010 Nov 24 PMID: 21102634).
Mansour WY, Tennstedt P, Volquardsen J, Oing C, Kluth M, Hube-Magg C, Borgmann K, Simon R, Petersen C, Dikomey E, Rothkamm K. Loss of PTEN-assisted G2/M checkpoint impedes homologous recombination repair and enhances radio-curability and PARP inhibitor treatment response in prostate cancer. Sci Rep. 2018;8(1):1–2.
Gong L, Govan JM, Evans EB, Dai H, Wang E, Lee SW, Lin HK, Lazar AJ, Mills GB, Lin SY. Nuclear PTEN tumor-suppressor functions through maintaining heterochromatin structure. Cell Cycle. 2015;14(14):2323–32.
He J, Zhang Z, Ouyang M, Yang F, Hao H, Lamb KL, Yang J, Yin Y, Shen WH. PTEN regulates EG5 to control spindle architecture and chromosome congression during mitosis. Nat Commun. 2016;5(7):12355. https://doi.org/10.1038/ncomms12355.PMID:27492783;PMCID:PMC4980451.
Feng J, Liang J, Li J, Li Y, Liang H, Zhao X, McNutt MA, Yin Y. PTEN controls the DNA replication process through MCM2 in response to replicative stress. Cell Rep. 2015;13(7):1295–303. https://doi.org/10.1016/j.celrep.2015.10.016.
Misra S, Mukherjee A, Karmakar P. Phosphorylation of PTEN at STT motif is associated with DNA damage response. Mutat Res. 2014;770:112–9.
Steelman LS, Navolanic PM, Sokolosky ML, Taylor JR, Lehmann BD, Chappell WH, Abrams SL, Wong EW, Stadelman KM, Terrian DM, Leslie NR, Martelli AM, Stivala F, Libra M, Franklin RA, McCubrey JA. Suppression of PTEN function increases breast cancer chemotherapeutic drug resistance while conferring sensitivity to mTOR inhibitors. Oncogene. 2008;27(29):4086–95. https://doi.org/10.1038/onc.2008.49.
Schmitz M, Grignard G, Margue C, Dippel W, Capesius C, Mossong J, Nathan M, Giacchi S, Scheiden R, Kieffer N. Complete loss of PTEN expression as a possible early prognostic marker for prostate cancer metastasis. Int J Cancer. 2007;120:1284–92. https://doi.org/10.1002/ijc.22359.
Da-M Li, Sun H. PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. PNAS. 1998;95(26):15406–11. https://doi.org/10.1073/pnas.95.26.15406.
Liu Y, Du X, Zhang S, Liu Y, Zhang Q, Yin Q, McNutt MA, Yin Y. PTEN regulates spindle assembly checkpoint timing through MAD1 in interphase. Oncotarget. 2017;8(58):98040–50. https://doi.org/10.18632/oncotarget.20532.PMID:29228672;PMCID:PMC5716712.
Choi BH, Xie S, Dai W. PTEN is a negative regulator of mitotic checkpoint complex during the cell cycle. Exp Hematol Oncol. 2017;29(6):19. https://doi.org/10.1186/s40164-017-0079-0.PMID:28670501;PMCID:PMC5492438.
van Ree JH, Nam HJ, Jeganathan KB, Kanakkanthara A, van Deursen JM. Pten regulates spindle pole movement through Dlg1-mediated recruitment of Eg5 to centrosomes. Nat Cell Biol. 2016;18(7):814–21. https://doi.org/10.1038/ncb3369.
Leonard MK, Hill NT, Bubulya PA, Kadakia MP. The PTEN-Akt pathway impacts the integrity and composition of mitotic centrosomes. Cell Cycle. 2013;12(9):1406–15. https://doi.org/10.4161/cc.24516.
Song MS, Carracedo A, Salmena L, Song SJ, Egia A, Malumbres M, Pandolfi pp. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell. 2011;144(2):187–99. https://doi.org/10.1016/j.cell.2010.12.020.
Acknowledgements
This work is financially supported by The Department of Science and Technology (DST-SERB), Government of India (Sanction No. EMR/2016/001151). Ginia Ghosh was awarded a fellowship from the Council of Scientific and Industrial Research (Sanction no. 37(1673)/16/EMR-II). Prof. Dr. W.R. Sellers of Harvard Medical School generously donated hosphor-deficient PTEN and wild-type PTEN. We also appreciate and thank Dr. Prosenjit Sen of the Indian Association for Science for providing confocal microscopy.
Funding
This study is supported by the Department of Science and Technology, Republic of India, EMR/2016/001151 to Parimal Karmakar.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghosh, G., Misra, S., Ray, R. et al. Phospho PTEN mediated dephosphorylation of mitotic kinase PLK1 and Aurora Kinase A prevents aneuploidy and preserves genomic stability. Med Oncol 40, 119 (2023). https://doi.org/10.1007/s12032-023-01985-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-01985-z