Abstract
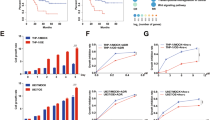
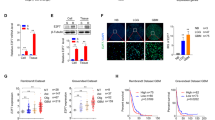
Although temozolomide is the primary chemotherapeutic agent in glioblastoma, current studies have focused on its combinational applications to overcome resistance by targeting multiple pathways. JAK/STAT and WNT are among the essential cancer-related signaling pathways. Ruxolitinib, the first approved JAK1/2 inhibitor, has promise in glioblastoma with its blood–brain barrier (BBB) crossing ability. The mentioned study aims to evaluate the anti-cancer potential of ruxolitinib individually and in combination with temozolomide on glioblastoma cells, brain cancer stem cells (BCSCs), and BBB-forming healthy cells. It also intends to determine the effects of JAK inhibitor treatment in combination with temozolomide on WNT signaling, which is known to cross-talk with the JAK/STAT pathway. The U87MG, BCSC, and HBMEC cell lines were the in vitro models. The cytotoxic and apoptotic effects of ruxolitinib and the combination were determined by the WST-1 test and Annexin V assay, respectively. The expression level changes of WNT signaling pathway genes caused by ruxolitinib and the combination treatments were defined by the qRT-PCR method. Network analysis of significantly upregulated and downregulated genes was performed via the GO KEGG pathway enrichment module of the String V11.5 database. The IC50 value of the ruxolitinib on U87MG glioblastoma cells was determined as 94.07 µM at 24th h. The combination of temozolomide and ruxolitinib had a synergistic effect on U87MG cells at 24th h. The combination index (CI) was determined as 0.796, and ED60 values of ruxolitinib and temozolomide were determined as 89.75 and 391.48 µM, respectively. Ruxolitinib improves the apoptotic effect of temozolomide on glioblastoma cells and brain cancer stem cells. Ruxolitinib regulates the WNT signaling pathway both individually and in combination with temozolomide. Our study indicates the potential of ruxolitinib to increase the cytotoxic and apoptotic activity of temozolomide in glioblastoma cells, also considering CSCs and healthy BBB-forming cells. As supported by gene expression and network analyses, the BBB-crossing agent ruxolitinib promises the potential to increase the efficacy of temozolomide in glioblastoma by affecting multiple signaling pathways in both cancer cells and CSCs.






Similar content being viewed by others
Data availability
All data are presented in the manuscript and supplementary data section.
References
Kanderi T, Gupta V. Glioblastoma multiforme. In Treasure Island (FL). J Biomed Res. 2022;37:1–1.
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–51.
Fisher JP, Adamson DC. Current FDA-approved therapies for high-grade malignant Gliomas. Biomedicines. 2021;9(3):324.
Singh N, Miner A, Hennis L, Mittal S. Mechanisms of temozolomide resistance in glioblastoma—a comprehensive review. Cancer Drug Resist. 2021;4(1):17–43.
Hegi ME, Diserens A-C, Gorlia T, Hamou M-F, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003.
Kuşoğlu A, Biray AÇ. Cancer stem cells: a brief review of the current status. Gene. 2019;681:80–5.
Bisht S, Nigam M, Kunjwal SS, Sergey P, Mishra AP, Sharifi-Rad J. Cancer stem cells: from an insight into the basics to recent advances and therapeutic targeting. Stem Cells Int. 2022;2022:9653244.
Kim SJ, Kang HG, Kim K, Kim H, Zetterberg F, Park YS, et al. Crosstalk between WNT and STAT3 is mediated by galectin-3 in tumor progression. Gastric Cancer. 2021;24(5):1050–62.
Fragoso MA, Patel AK, Nakamura REI, Yi H, Surapaneni K, Hackam AS. The Wnt/β-catenin pathway cross-talks with STAT3 signaling to regulate survival of retinal pigment epithelium cells. PLoS One. 2012;7(10):e46892.
Bagca BG, Ozalp O, Kurt CC, Mutlu Z, Saydam G, Gunduz C, et al. Ruxolitinib induces autophagy in chronic myeloid leukemia cells. Tumor Biol. 2016;37(2):1573–9.
Kusoglu A, Bagca BG, Ozates Ay NP, Saydam G, Avci CB. Ruxolitinib regulates the autophagy machinery in multiple myeloma cells. Anticancer Agents Med Chem. 2020;20(18):2316–23.
Goker Bagca B, Biray AC. The potential of JAK/STAT pathway inhibition by ruxolitinib in the treatment of COVID-19. Cytokine Growth Fact Rev. 2020;54:51–61.
Haile WB, Gavegnano C, Tao S, Jiang Y, Schinazi RF, Tyor WR. The Janus kinase inhibitor ruxolitinib reduces HIV replication in human macrophages and ameliorates HIV encephalitis in a murine model. Neurobiol Dis. 2016;92:137–43.
Rauf Y, Hufsey R, Robinson K, Suh JH, Chao ST, Murphy ES, et al. Phase I study of ruxolitinib with radiation and temozolomide in patients with newly diagnosed grade III gliomas and glioblastoma. J Clin Oncol. 2021;2060:60.
Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49(1):605–12.
Abbaszade Z, Bagca BG, Avci CB. Molecular biological investigation of temozolomide and KC7F2 combination in U87MG glioma cell line. Gene. 2021;776:145445.
Brooks AJ, Putoczki T. Jak-stat signalling pathway in cancer. Cancers. 2020;12(7):1971.
Civallero M, Cosenza M, Pozzi S, Sacchi S. Ruxolitinib combined with vorinostat suppresses tumor growth and alters metabolic phenotype in hematological diseases. Oncotarget. 2017;8(61):103797.
Yilmaz CU, Bagca BG, Karaca E, Durmaz A, Durmaz B, Aykut A, et al. Propolis extract regulates microrna expression in glioblastoma and brain cancer stem cells. Anticancer Agents Med Chem. 2021;22(2):378–89.
Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36(11):1461–73.
Covey TM, Kaur S, Ong T, Proffitt KD, Wu Y, Tan P, et al. PORCN moonlights in a wnt-independent pathway that regulates cancer cell proliferation. PLoS ONE. 2012;7(4):e34532.
Latour M, Her NG, Kesari S, Nurmemmedov E. WNT signaling as a therapeutic target for glioblastoma. Int j mol sci. 2021;22(16):8428.
Martinez-Font E, Pérez-Capó M, Ramos R, Felipe I, Garcías C, Luna P, et al. Impact of wnt/β-catenin inhibition on cell proliferation through cdc25a downregulation in soft tissue sarcomas. Cancers (Basel). 2020;12(9):2556.
Tao W, Chu C, Zhou W, Huang Z, Zhai K, Fang X, et al. Dual Role of WISP1 in maintaining glioma stem cells and tumor-supportive macrophages in glioblastoma. Nat Commun. 2020;11(1):1–6.
Stuelten CH, Parent CA, Montell DJ. Cell motility in cancer invasion and metastasis: ınsights from simple model organisms. Nat Rev Cancer. 2018;18(5):296–312.
Parker JJ, Canoll P, Niswander L, Kleinschmidt-DeMasters BK, Foshay K, Waziri A. Intratumoral heterogeneity of endogenous tumor cell invasive behavior in human glioblastoma. Sci Rep. 2018;8(1):1.
Schcolnik-Cabrera A, Juárez-López D. Dual contribution of the mTOR pathway and of the metabolism of amino acids in prostate cancer. Cell Oncol (Dordr). 2022;1:29.
Drakulic D, Schwirtlich M, Petrovic I, Mojsin M, Milivojevic M, Kovacevic-Grujicic N, et al. Current opportunities for targeting dysregulated neurodevelopmental signaling pathways in glioblastoma. Cells. 2022;11:16.
Clark PA, Iida M, Treisman DM, Kalluri H, Ezhilan S, Zorniak M, et al. Activation of multiple ERBB family receptors mediates glioblastoma cancer stem-like cell resistance to EGFR-targeted inhibition. Neoplasia (United States). 2012;14(5):420–513.
Xu K, Zhang Z, Pei H, Wang H, Li L, Xia Q. FoxO3a induces temozolomide resistance in glioblastoma cells via the regulation of β-catenin nuclear accumulation. Oncol Rep. 2017;37(4):2391–7.
Hung HC, Liu CC, Chuang JY, Su CL, Gean PW. Inhibition of sonic hedgehog signaling suppresses glioma stem-like cells likely through ınducing autophagic cell death. Front Oncol. 2020;10:1233.
Zheng Q, Diao S, Wang Q, Zhu C, Sun X, Yin B, et al. IL-17A promotes cell migration and invasion of glioblastoma cells via activation of PI3K/AKT signalling pathway. J Cell Mol Med. 2019;23(1):357–69.
Asad AS, Nicola Candia AJ, Gonzalez N, Zuccato CF, Abt A, Orrillo SJ, et al. Prolactin and its receptor as therapeutic targets in glioblastoma multiforme. Sci Rep. 2019;9(1):1–6.
Stamatovic SM, Johnson AM, Keep RF, Andjelkovic AV. Junctional proteins of the blood-brain barrier: new insights into function and dysfunction. Tissue Barriers. 2016;4(1):e1154641.
Deisseroth A, Kaminskas E, Grillo J, Chen W, Saber H, Lu HL, et al. US food and drug administration approval: ruxolitinib for the treatment of patients with intermediate and high-risk myelofibrosis. Clin Cancer Res. 2012;18(12):3212–7.
Chaudhuri A, Yang B, Gendelman HE, Persidsky Y, Kanmogne GD. STAT1 signaling modulates HIV-1-induced inflammatory responses and leukocyte transmigration across the blood-brain barrier. Blood. 2008;18(12):3212–7.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goker Bagca, B., Ozates, N.P. & Biray Avci, C. Ruxolitinib enhances cytotoxic and apoptotic effects of temozolomide on glioblastoma cells by regulating WNT signaling pathway-related genes. Med Oncol 40, 37 (2023). https://doi.org/10.1007/s12032-022-01897-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01897-4