Abstract
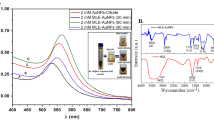
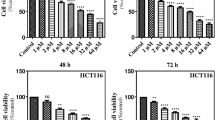
In prior studies, Quercetin was revealed to exhibit anti-cancer features in a variety of cancer cell lines. However, the impact of Quercetin on neuroblastoma is unknown. This study looked into the potential cytotoxic effects of Quercetin and Quercetin-loaded chitosan nanoparticles (NPs) on the SH-SY5Y cell line. In this study, NPs containing Quercetin was prepared and characterization studies were performed. The vitality of the cells was measured using the XTT test after 24 h of treatment with various concentrations of Quercetin (0.5, 1, 2, 4, and 8 µg/mL). ELISA kits were used to detect the amounts of cleaved PARP, BCL-2, 8-Hydroxy-deoxyguanosine (8-oxo-dG), cleaved caspase 3, Bax, total oxidant status, and total antioxidant status in the cells. The results of the chitosan NPs characterization investigation revealed that the particle size, encapsulation effectiveness, and drug release profile of NPs were all appropriate for cell culture studies. Quercetin and Quercetin-loaded chitosan NPs significantly reduced cell viability in SH-SY5Y cells at different concentrations (**p < 0.05). 2 µg/mL Quercetin and Quercetin-loaded chitosan NPs significantly enhanced the levels of 8-oxo-dG, cleaved caspase 3, Bax, cleaved PARP, and total oxidant in ELISA testing. However, treatment with 2 µg/mL of Quercetin and Quercetin-loaded chitosan NPs did not affect the amount of BCL-2 protein. Overall, Quercetin and Quercetin-loaded chitosan NPs caused significant cytotoxicity in SH-SY5Y cells via producing oxidative stress, DNA damage, and eventually apoptosis.






Similar content being viewed by others
Data availability
All the data generated or analyzed during this study are included in this manuscript.
References
Docea AO, Mitrut P, Grigore D, Pirici D, Calina DC, Gofita E. Immunohistochemical expression of TGF beta (TGF-beta), TGF beta receptor 1 (TGFBR1), and Ki67 in intestinal variant of gastric adenocarcinomas. Rom J Morphol Embryol. 2012;53(3):683–92.
Salehi B, Jornet PL, Lopez EPF, Calina D, Sharifi-Rad M, Ramirez-Alarcon K, Forman K, Fernandez M, Martorell M, Setzer WN, et al. Plant-derived bioactives in oral mucosal lesions: a key emphasis to curcumin, lycopene, chamomile, aloe vera green tea and coffee properties. Biomolecules. 2019;9(3):1–23.
Sharifi-Rad M, Kumar NVA, Zucca P, Varoni EM, Dini L, Panzarini E, Rajkovic J, Fokou PVT, Azzini E, Peluso I, et al. Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol. 2020;11:1–21.
Ghad A, Mahjoub S, Tabandeh F, Talebnia F. Synthesis and optimization of chitosan nanoparticles: potential applications in nanomedicine and biomedical engineering. Caspian J Intern Med. 2014;5:156–61.
Park W, Heo YJ, Han DK. New opportunities for nanoparticles in cancer immunotherapy. Biomater Res. 2018;22:24–33. https://doi.org/10.1186/s40824-018-0133-y.
Jovčevska I, Muyldermans S. The therapeutic potential of nanobodies. BioDrugs Clin Immunotherap Biopharm Gene Therapy. 2020;34(1):11–26. https://doi.org/10.1007/s40259-019-00392-z.
Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8(1):59–73. https://doi.org/10.1038/nri2216.
Wang R, Billone PS, Mullett WM. Nanomedicine in action: an overview of cancer nanomedicine on the market and in clinical trials. J Nanomater. 2013;2013:1–12.
Adair JH, Parette MP, Altinoglu EI, Kester M. Nanoparticulate alternatives for drug delivery. ACS Nano. 2010;4(9):4967–70.
Altinoglu EI, Adair JH. Near infrared imaging with nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(5):461–77.
Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Drug Resist. 2019;2:141–60.
Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The different mechanisms of cancer drug resistance: a brief review. Tabriz Univ Med Sci. 2017;7:339–48.
Longacre M, Snyder N, Sarkar S. Drug resistance in cancer: an overview. Cancers (Basel). 2014;6:1769–92.
Xue X, Liang XJ. Overcoming drug efflux-based multidrug resistance in cancer with nanotechnology. Chin J Cancer. 2012;31:100–9.
Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of efflux pumps in multidrug-resistant cancer. Nat Rev Cancer. 2018;18:452–64.
Singh A, Benjakul S, Prodpran T. Ultrasound assisted extraction of chitosan from squid pen: molecular characterization and fat binding capacity. J Food Sci. 2019;84:224–34.
Mittal A, Singh A, Benjakul S, Prodpran T, Nilsuwan K, Huda N, Caba KDL. Composite films based on chitosan and epigallocatechin gallate grafted chitosan: characterization, antioxidant and antimicrobial activities. Food Hydrocol. 2020;111:1–10.
Singh A, Benjakul S, Prodpran T. Chitooligosaccharides from squid pen prepared using different enzymes: characteristics and the effect on quality of surimi gel during refrigerated storage. Food Prod Process Nutri. 2019;1:1–10.
Li J, Cai C, Ja L. Chitosan-based nanomaterials for drug delivery. Molecules. 2018;23:2661. https://doi.org/10.3390/molecules23102661.
Bhattarai N, Gunn J, Zhang M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev. 2010;62(1):83–99. https://doi.org/10.1016/j.addr.2009.07.019.
Torabi N, Dobakhti F, Faghihzadeh S, Haniloo A. In vitro and in vivo effects of chitosan-praziquantel and chitosan-albendazole nanoparticles on Echinococcus granulosus Metacestodes. Parasitol Res. 2018;117:2015–23. https://doi.org/10.1007/s00436.
Jhaveri J, Raichura Z, Khan T, Momin M, Omri A. Chitosan nanoparticles-insight into properties, functionalization and applications in drug delivery and theranostics. Molecules. 2021;26:272. https://doi.org/10.3390/molecules26020272.
Cheimonidi C, Samara P, Polychronopoulos P, et al. Selective cytotoxicity of the herbal substance acteoside against tumor cells and its mechanistic insights. Redox Biol. 2018;16:169–78.
Tavana E, Mollazadeh H, Mohtashami E, et al. Quercetin: A promising phytochemical for the treatment of glioblastoma multiforme. BioFactors. 2020;46:356–66.
Taskin T, Dogan M, Yilmaz BN, Senkardes I. Phytochemical screening and evaluation of antioxidant, enzyme inhibition, anti-proliferative and calcium oxalate anti-crystallization activities of Micromeria fruticosa spp. brachycalyx and Rhus coriaria. Biocatal Agric Biotechnol. 2020;27:1–7.
Calvo P, Remunan-Lopez C, Vila-Jato JL, Alonso MJ. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci. 1997;63(1):125–32.
Wikanta T, Erizal T, Tjahyono T, Sugiyono T. Synthesis of polyvinyl alcohol-chitosan hydrogel and study of its swelling and antibacterial properties. Squalen Bull Mar Fish Postharvest Biotechnol. 2012;7(1):1–10.
Purbowatiningrum N, Ismiyarto EF. Cinnamomum casia extract encapsulated nanochitosan as antihypercholesterol. IOP Conf Ser: Mater Sci Eng. 2017;172:012035.
Han HJ, Lee JS, Park SA, Ahn JB, Lee HG. Extraction optimization and nanoencapsulation of jujube pulp and seed for enhancing antioxidant activity. Colloids Surf B. 2015;130:93–100.
Keawchaoon L, Yoksan R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf B Biointerfaces. 2011;84:163–71.
Mohammadi A, Hashemi M, Hosseini S. Chitosan nanoparticles loaded with Cinnamomum zeylanicum essential oil enhance the shelf life of cucumber during cold storage. Postharvest Biol Technol. 2015;110:203–13.
Taşkın D, Doğan M, Ermanoğlu M, Arabaci T. Achillea goniocephala extract loaded into nanochitosan: in vitro cytotoxic and antioxidant activity. Clin Exp Health Sci. 2021;11(4):659–66.
Doğan M, Karademir M. Effect of captopril on the oxidative damage caused by pentylenetetrazole in the SHSY-5Y human neuroblastoma cell line. Cumhuriyet Med J. 2020;42(4):479–83.
Tang H, Zhang Y, Li D, et al. Discovery and synthesis of novel magnolol derivatives with potent anticancer activity in non-small cell lung cancer. Eur J Med Chem. 2018;156:190–205.
Chen J. Recent advance in the studies of β-glucans for cancer therapy. Anticancer Agents Med Chem. 2013;13:679–80.
Sachdev E, Tabatabai R, Roy V, Rimel BJ, Mita MM. PARP Inhibition in cancer: An update on clinical development. Target Oncol. 2019;14:657–79.
Sima P, Richter J, Vetvicka V. Glucans as new anticancer agents. Anticancer Res. 2019;39:3373–8.
Guo C, Li X, Wang R, et al. Association between oxidative DNA damage and risk of colorectal cancer: sensitive determination of urinary 8-hydroxy-2′-deoxyguanosine by UPLC-MS/MS analysis. Sci Rep. 2016;6:1–9. https://doi.org/10.1038/srep32581.
Chen Z, Zhang B, Gao F, Shi R. Modulation of G2/M cell cycle arrest and apoptosis by luteolin in human colon cancer cells and xenografts. Oncol Lett. 2018;15:1559–65. https://doi.org/10.3892/ol.2017.7475.
Lee SI, Jeong YJ, Yu AR, Kwak HJ, Cha JY, Kang I, Yeo EJ. Carfilzomib enhances cisplatin-induced apoptosis in SK-NBE(2)-M17 human neuroblastoma cells. Sci Rep. 2019;9:1–14.
Filiz AK, Joha Z, Yulak F. Mechanism of anti-cancer effect of β-glucan on SH-SY5Y cell line. Bangladesh J Pharmacol. 2021;16(4):122–8.
Acknowledgements
This study was performed by own expense in Cumhuriyet University Faculty of Pharmacy Laboratory of Pharmaceutical Biotechnology and Cumhuriyet University Faculty of Medicine Research Center.
Funding
The author has not disclosed any funding.
Author information
Authors and Affiliations
Contributions
MD performed experiments and analyzed data. MD supervised the entire project and designed the experiments. MD wrote the paper and read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dogan, M. Assessment of mechanism involved in the apoptotic and anti-cancer activity of Quercetin and Quercetin-loaded chitosan nanoparticles. Med Oncol 39, 176 (2022). https://doi.org/10.1007/s12032-022-01820-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01820-x