Abstract
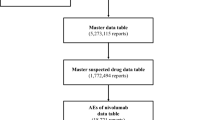
The present study aimed to determine the risk of trastuzumab-induced lung toxicity, time to onset, and post hoc outcomes using the Japanese Adverse Drug Event Report database. We analyzed data for the period between April 2004 and March 2021. Data on lung toxicities were extracted, and relative risk of adverse events (AEs) was estimated using the reporting odds ratio. We analyzed 1,772,494 reports and identified 4362 reports of AEs caused by trastuzumab. Of these, 693 lung toxicities were reportedly associated with trastuzumab. Signals were detected for seven lung toxicities: interstitial lung disease, pulmonary edema, pleural effusion, lung disorder, acute pulmonary edema, pulmonary fibrosis, and radiation pneumonitis. Among these, interstitial lung disease was the most frequently reported (61.8%). A histogram of times to onset showed occurrence from 1 to 105 days, but some cases of interstitial lung disease occurred even more than one year after the start of administration. The AEs showing the highest fatality rates were interstitial lung disease, pulmonary fibrosis, and radiation pneumonitis. This study focused on lung toxicities caused by trastuzumab as post-marketing AEs. Some cases could potentially involve serious outcomes; therefore, patients should be monitored for signs of the onset of these AEs not only at the start of administration, but also over an extended period, especially for interstitial lung disease.



Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. https://doi.org/10.1056/NEJM200103153441101.
Eiermann W, International Herceptin Study Group. Trastuzumab combined with chemotherapy for the treatment of HER2-positive metastatic breast cancer: pivotal trial data. Ann Oncol. 2001;12(Suppl 1):S57-62.
Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–26. https://doi.org/10.1200/JCO.2002.20.3.719.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. https://doi.org/10.1016/S0140-6736(10)61121-X.
Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, VanderWalde A, et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019;20:518–30. https://doi.org/10.1016/S1470-2045(18)30904-5.
Takahashi H, Tada Y, Saotome T, Akazawa K, Ojiri H, Fushimi C, et al. Phase II trial of trastuzumab and docetaxel in patients with human epidermal growth factor receptor 2-positive salivary duct carcinoma. J Clin Oncol. 2019;37:125–34. https://doi.org/10.1200/JCO.18.00545.
Chen T, Xu T, Li Y, Liang C, Chen J, Lu Y, et al. Risk of cardiac dysfunction with trastuzumab in breast cancer patients: a meta-analysis. Cancer Treat Rev. 2011;37:312–20. https://doi.org/10.1016/j.ctrv.2010.09.001.
Thompson LM, Eckmann K, Boster BL, Hess KR, Michaud LB, Esteva FJ, et al. Incidence, risk factors, and management of infusion-related reactions in breast cancer patients receiving trastuzumab. Oncologist. 2014;19:228–34. https://doi.org/10.1634/theoncologist.2013-0286.
Long HD, Lin YE, Zhang JJ, Zhong WZ, Zheng RN. Risk of congestive heart failure in early breast cancer patients undergoing adjuvant treatment with trastuzumab: a meta-analysis. Oncologist. 2016;21:547–54. https://doi.org/10.1634/theoncologist.2015-0424.
Rossi M, Carioli G, Bonifazi M, Zambelli A, Franchi M, Moja L, et al. Trastuzumab for HER2+ metastatic breast cancer in clinical practice: cardiotoxicity and overall survival. Eur J Cancer. 2016;52:41–9. https://doi.org/10.1016/j.ejca.2015.09.012.
Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz C, Virgo KS, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25:3991–4008. https://doi.org/10.1200/JCO.2007.10.9777.
Pivot X, Suter T, Nabholtz JM, Pierga JY, Espie M, Lortholary A, et al. Cardiac toxicity events in the PHARE trial, an adjuvant trastuzumab randomised phase III study. Eur J Cancer. 2015;51:1660–6. https://doi.org/10.1016/j.ejca.2015.05.028.
Uchida M, Kondo Y, Suzuki S, Hosohata K. Evaluation of acute kidney injury associated with anticancer drugs used in gastric cancer in the Japanese Adverse Drug Event Report database. Ann Pharmacother. 2019;53:1200–6. https://doi.org/10.1177/1060028019865870.
Sugawara H, Uchida M, Suzuki S, Suga Y, Uesawa Y, Nakagawa T, et al. Analyses of respiratory depression associated with opioids in cancer patients based on the Japanese Adverse Drug Event report database. Biol Pharm Bull. 2019;42:1185–91. https://doi.org/10.1248/bpb.b19-00105.
Uchida M, Kawashiri T, Maegawa N, Takano A, Hosohata K, Uesawa Y. Pharmacovigilance evaluation of bendamustine-related skin disorders using the Japanese Adverse Drug Event Report database. J Pharm Sci. 2021;24:16–22. https://doi.org/10.18433/jpps31597.
Nakao S, Uchida M, Satoki A, Okamoto K, Uesawa Y, Shimizu T. Evaluation of cardiac adverse events associated with carfilzomib using a Japanese real-world database. Oncology. 2022;100:60–4. https://doi.org/10.1159/000519687.
van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11:3–10. https://doi.org/10.1002/pds.668.
Ando G, Taguchi K, Enoki Y, Yokoyama Y, Kizu J, Matsumoto K. Evaluation of the expression time of ganciclovir-induced adverse events using JADER and FAERS. Biol Pharm Bull. 2019;42:1799–804. https://doi.org/10.1248/bpbb19-00156.
Sauzet O, Carvajal A, Escudero A, Molokhia M, Cornelius VR. Illustration of the Weibull shape parameter signal detection tool using electronic healthcare record data. Drug Saf. 2013;36:995–1006. https://doi.org/10.1007/s40264-013-0061-7.
Hirooka T, Yamada M (2012) Evaluation of risk of adverse reaction using PMDA ‘Adverse Drug Reaction Database. Database SAS User Groups Ronbun-syu
Sugaya A, Ishiguro S, Mitsuhashi S, Abe M, Hashimoto I, Kaburagi T, et al. Interstitial lung disease associated with trastuzumab monotherapy: a report of 3 cases. Mol Clin Oncol. 2017;6:229–32. https://doi.org/10.3892/mco.2016.1113.
Abulkhair O, El Melouk W. Delayed paclitaxel-trastuzumab-induced interstitial pneumonitis in breast cancer. Case Rep Oncol. 2011;4:186–91. https://doi.org/10.1159/000326063.
Hackshaw MD, Danysh HE, Singh J, Ritchey ME, Ladner A, Taitt C, et al. Incidence of pneumonitis/interstitial lung disease induced by HER2-targeting therapy for HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2020;183:23–39. https://doi.org/10.1007/s10549-020-05754-8.
Teuwen LA, Van den Mooter T, Dirix L. Management of pulmonary toxicity associated with targeted anticancer therapies. Expert Opin Drug Metab Toxicol. 2015;11:1695–707. https://doi.org/10.1517/17425255.2015.1080687.
Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–21. https://doi.org/10.1056/NEJMoa1914510.
Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382:2419–30. https://doi.org/10.1056/NEJMoa2004413.
Acknowledgements
We are grateful to Professor Yoshihiro Uesawa from the Department of Medical Molecular Informatics, Meiji Pharmaceutical University. We are also grateful to Tadashi Hirooka (TAIHO PHARMA Corporation) for his lecture on Hirooka methods using the JADER database.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
YK and MU contributed to data curation; writing of the original draft; and writing, reviewing, and editing of the manuscript. MK and HA contributed to data curation and writing, reviewing, and editing of the manuscript. TS contributed to conceptualisation; supervision; and writing, reviewing, and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
All authors declare no conflict of interest.
Ethical approval
Ethics approval was not sought for this study; given the database-related, observational design without direct involvement of any research subjects. All results were obtained from data openly available online from the PMDA website (www.pmda.go.jp). All data from the JADER database were fully anonymized by the relevant regulatory authority before we accessed them. Thus, all methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kanbayashi, Y., Uchida, M., Kashiwagi, M. et al. Evaluation of lung adverse events with trastuzumab using the Japanese pharmacovigilance database. Med Oncol 39, 219 (2022). https://doi.org/10.1007/s12032-022-01805-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01805-w