Abstract
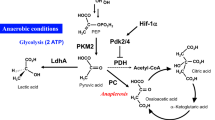
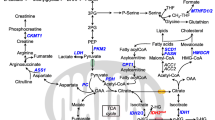
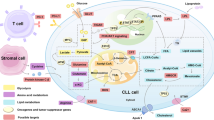
Chronic myeloid leukemia (CML) is characterized by the possession of the Philadelphia chromosome, which contains the Bcr-Abl oncogene that codes for the oncoprotein BCR-ABL. Through glucose metabolism, glycolysis, and the translocation of the high-affinity glucose transporter to the cell surface, BCR-ABL modulates various signaling pathways in CML cells and maintains ATP turnover in tumor cells. Given the effective results of anti-tumor drugs in normalizing abnormal cellular metabolism, Imatinib (IM) has begun to be investigated and proven to be a highly potent tyrosine kinase inhibitor (TKI) in CML therapy. Initially, IM was tested for aberrant glucose metabolism, but all four metabolisms (glucose, lipid, amino acid, and nucleotide) are interrelated and enhance tumor growth under stress; eventually, the other three metabolisms were investigated. Subsequent effects of IM therapy showed a switch from glycolysis to the tricarboxylic acid cycle, upregulation of pentose phosphate pathway-associated oxidative pathways, and internal translocation of glucose transporters. In terms of lipid metabolism, IM had contradictory results: in one study, it served as a triglyceride and total cholesterol regulator, while in another study, it had no impact. The effect of IM on altered amino acid and nucleotide metabolisms was investigated using a multi-omics approach, which revealed a decrease in sulfur-containing amino acids, aromatic amino acids, and nucleotide biosynthesis. So, despite the mixed effect on cellular metabolism, IM has more positive effects, and therefore, the drug proved to be better than other TKIs. The present study is one approach to determine the transformative activities of IM against CML-associated metabolic changes, but further investigation is still needed to uncover more potentials of IM.



Similar content being viewed by others
Data availability
No datasets were generated for the preparation of this manuscript.
References
Soverini S, Bassan R, Lion T. Treatment and monitoring of Philadelphia chromosome-positive leukemia patients: recent advances and remaining challenges. J Hematol Oncol. 2019. https://doi.org/10.1186/s13045-019-0729-2.
Tridente G. Adverse events. In: Adverse events and oncotargeted kinase inhibitors. Amsterdam: Elsevier; 2017.
Singh P, Kumar V, Gupta SK, Kumari G, Verma M. Combating TKI resistance in CML by inhibiting the PI3K/Akt/mTOR pathway in combination with TKIs: a review. Med Oncol. 2021;38(1):1–16. https://doi.org/10.1007/s12032-021-01462-5.
Singh P, Gupta SK, Ali V, Verma M. Downregulation of Bcr-Abl oncogene in chronic myeloid leukemia by micro RNAs. Asian Pac J Health Sci. 2018;5(4):65–84. https://doi.org/10.21276/apjhs.2018.5.4.12.
Rossari F, Minutolo F, Orciuolo E. Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy. J Hematol Oncol. 2018;11(1):1–14. https://doi.org/10.1186/s13045-018-0624-2.
Buchdunge E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-Kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000;295(1):139–45.
Demetri GD. Identification and treatment of chemoresistant inoperable or metastatic GIST: experience with the selective tyrosine kinase inhibitor imatinib mesylate (STI571). Eur J Cancer. 2002;38:S52–9. https://doi.org/10.1016/S0959-8049(02)80603-7.
Krystal GW. Imatinib mesylate (STI571) for myeloid malignancies other than CML. Leuk Res. 2004;28(1):53–9. https://doi.org/10.1016/j.leukres.2003.10.003.
de Kogel CE, Schellens JHM. Imatinib. Oncologist. 2007;12(12):1390–4. https://doi.org/10.1634/theoncologist.12-12-1390.
Vaidya S, Ghosh K, Vundinti BR. Recent developments in drug resistance mechanism in chronic myeloid leukemia: a review. Eur J Haematol. 2011. https://doi.org/10.1111/j.1600-0609.2011.01689.x.
Fava C, Rege-Cambrin G, Saglio G. Imatinib: the first-line CML therapy. Cham: Springer; 2021. p. 49–59.
Hughes T, White D. Which TKI? An embarrassment of riches for chronic myeloid leukemia patients. Hematology. 2013;2013(1):168–75. https://doi.org/10.1182/asheducation-2013.1.168.
Eskazan AE, Ozmen D. Tyrosine kinase inhibitor (TKI) therapy for newly-diagnosed patients with chronic myeloid leukemia: focusing on TKI discontinuation due to adverse events—is better always good? Expert Rev Hematol. 2017;10(7):583–6. https://doi.org/10.1080/17474086.2017.1339599.
Braun TP, Eide CA, Druker BJ. Response and resistance to BCR-ABL1-targeted therapies. Cancer Cell. 2020;37(4):530–42. https://doi.org/10.1016/j.ccell.2020.03.006.
Kumar V, Singh P, Gupta SK, Ali V, Verma M. Transport and metabolism of tyrosine kinase inhibitors associated with chronic myeloid leukemia therapy: a review. Mol Cell Biochem. 2022. https://doi.org/10.1007/s11010-022-04376-6.
Serkova N, Boros LG. Detection of resistance to imatinib by metabolic profiling. Am J Pharmacogenomics. 2005;5(5):293–302. https://doi.org/10.2165/00129785-200505050-00002.
Zhao Y, Butler EB, Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013;4(3):e532–e532. https://doi.org/10.1038/cddis.2013.60.
Weinhouse S, Warburg O, Burk D, Schade AL. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–70. https://doi.org/10.1126/science.124.3215.267.
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7(1):11–20. https://doi.org/10.1016/j.cmet.2007.10.002.
Lum JJ, Bui T, Gruber M, Gordan JD, DeBerardinis RJ, Covello KL, et al. The transcription factor HIF-1 plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21(9):1037–49. https://doi.org/10.1101/gad.1529107.
Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care. 2006;9(4):358–65. https://doi.org/10.1097/01.mco.0000232894.28674.30.
Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35. https://doi.org/10.1038/nrm3025.
Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8(3):224–36. https://doi.org/10.1016/j.cmet.2008.07.007.
Han H-S, Kang G, Kim JS, Choi BH, Koo S-H. Regulation of glucose metabolism from a liver-centric perspective. Exp Mol Med. 2016;48(3):e218–e218. https://doi.org/10.1038/emm.2015.122.
Tozzi M, Hansen JB, Novak I. Pannexin-1 mediated ATP release in adipocytes is sensitive to glucose and insulin and modulates lipolysis and macrophage migration. Acta Physiol. 2020;228(2):e13360. https://doi.org/10.1111/apha.13360.
Jaiswal N, Gavin MG, Quinn WJ, Luongo TS, Gelfer RG, Baur JA, et al. The role of skeletal muscle Akt in the regulation of muscle mass and glucose homeostasis. Mol Metab. 2019;28:1–13. https://doi.org/10.1016/j.molmet.2019.08.001.
Kominsky DJ, Klawitter J, Brown JL, Boros LG, Melo JV, Eckhardt SG, et al. Abnormalities in glucose uptake and metabolism in imatinib-resistant human BCR-ABL–positive cells. Clin Cancer Res. 2009;15(10):3442–50. https://doi.org/10.1158/1078-0432.CCR-08-3291.
Barnes K, McIntosh E, Whetton AD, Daley GQ, Bentley J, Baldwin SA. Chronic myeloid leukaemia: an investigation into the role of Bcr-Abl-induced abnormalities in glucose transport regulation. Oncogene. 2005;24(20):3257–67. https://doi.org/10.1038/sj.onc.1208461.
Ko BW, Han J, Heo JY, Jang Y, Kim SJ, Kim J, et al. Metabolic characterization of imatinib-resistant BCR-ABL T315I chronic myeloid leukemia cells indicates down-regulation of glycolytic pathway and low ROS production. Leuk Lymphoma. 2016;57(9):2180–8. https://doi.org/10.3109/10428194.2016.1142086.
Yan T-Y, Naren D-L, Gong Y-P. The roles of Glut5 in imatinib resistance in the Ph+ acute lymphoblastic leukemia cell. J Sichuan Univ Med Sci Ed. 2017;48(3):389–93.
Gottschalk S, Anderson N, Hainz C, Eckhardt SG, Serkova NJ. Imatinib (STI571)-mediated changes in glucose metabolism in human leukemia BCR-ABL-positive cells. Clin Cancer Res. 2004;10(19):6661–8. https://doi.org/10.1158/1078-0432.CCR-04-0039.
Boren J, Cascante M, Marin S, Comı́n-Anduix B, Centelles JJ, Lim S, et al. Gleevec (STI571) influences metabolic enzyme activities and glucose carbon flow toward nucleic acid and fatty acid synthesis in myeloid tumor cells. J Biol Chem. 2001;276(41):37747–53. https://doi.org/10.1074/jbc.M105796200.
Hagerkvist R, Makeeva N, Elliman S, Welsh N. Imatinib mesylate (Gleevec) protects against streptozotocin-induced diabetes and islet cell death in vitro. Cell Biol Int. 2006;30(12):1013–7. https://doi.org/10.1016/j.cellbi.2006.08.006.
Wolf AM, Wolf D, Rumpold H, Ludwiczek S, Enrich B, Gastl G, et al. The kinase inhibitor imatinib mesylate inhibits TNF-α production in vitro and prevents TNF-dependent acute hepatic inflammation. PNAS. 2005;102(38):13622–7. https://doi.org/10.1073/pnas.0501758102.
Louvet C, Szot GL, Lang J, Lee MR, Martinier N, Bollag G, et al. Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. PNAS. 2008;105(48):18895–900. https://doi.org/10.1073/pnas.0810246105.
Han MS, Chung KW, Cheon HG, Rhee SD, Yoon C-H, Lee M-K, et al. Imatinib mesylate reduces endoplasmic reticulum stress and induces remission of diabetes in db/db mice. Diabetes. 2009;58(2):329–36. https://doi.org/10.2337/db08-0080.
Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes. JAMA. 2009;302(2):179–218. https://doi.org/10.1001/jama.2009.976.
Fitter S, Vandyke K, Schultz CG, White D, Hughes TP, Zannettino ACW. Plasma adiponectin levels are markedly elevated in imatinib-treated chronic myeloid leukemia (CML) patients: a mechanism for improved insulin sensitivity in type 2 diabetic CML patients? J Clin Endocrinol Metab. 2010;95(8):3763–7. https://doi.org/10.1210/jc.2010-0086.
Zdenek R, Belohlavkova P, Cetkovsky P, Faber E, Klamova H, Ludmila M, et al. Comparison of glucose and lipid metabolism abnormality during nilotinib, imatinib and dasatinib therapy—results of enigma 2 study. Blood. 2014;124(21):1813. https://doi.org/10.1182/blood.V124.21.1813.1813.
Hosch SE, Olefsky JM, Kim JJ. Applied mechanics: uncovering how adiponectin modulates insulin action. Cell Metab. 2006;4(1):5–6. https://doi.org/10.1016/j.cmet.2006.06.003.
Fitter S, Vandyke K, Gronthos S, Zannettino ACW. Suppression of PDGF-induced PI3 kinase activity by imatinib promotes adipogenesis and adiponectin secretion. J Mol Endocrinol. 2012;48(3):229–40. https://doi.org/10.1530/JME-12-0003.
Wijesekara N, Krishnamurthy M, Bhattacharjee A, Suhail A, Sweeney G, Wheeler MB. Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J Biol Chem. 2010;285(44):33623–31. https://doi.org/10.1074/jbc.M109.085084.
Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol. 2010;55:207–25. https://doi.org/10.1146/annurev-ento-112408-085356.
Lehninger AL, Nelson DL, Cox MM, Cox MM. Lehninger principles of biochemistry. New York: Macmillan; 2005.
Jo Y, Okazaki H, Moon Y-A, Zhao T. Regulation of lipid metabolism and beyond. Int J Endocrinol. 2016;2016:1. https://doi.org/10.1155/2016/5415767.
Harris JR. Cholesterol binding and cholesterol transport proteins. Dordrecht: Springer; 2010.
Feingold KR, Grunfeld C. Introduction to lipids and lipoproteins. South Dartmouth: MDText.com inc; 2000.
Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7(10):763–77. https://doi.org/10.1038/nrc2222.
Lassila M, Allen TJ, Cao Z, Thallas V, Jandeleit-Dahm KA, Candido R, et al. Imatinib attenuates diabetes-associated atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(5):935–42. https://doi.org/10.1161/01.ATV.0000124105.39900.db.
Iurlo A, Orsi E, Cattaneo D, Resi V, Bucelli C, Orofino N, et al. Effects of first- and second-generation tyrosine kinase inhibitor therapy on glucose and lipid metabolism in chronic myeloid leukemia patients: a real clinical problem? Oncotarget. 2015;6(32):33944. https://doi.org/10.18632/oncotarget.5580.
Gologan R, Constantinescu G, Georgescu D, Ostroveanu D, Vasilache D, Dobrea C, et al. Hypolipemiant besides antileukemic effect of imatinib mesylate. Leuk Res. 2009;33(9):1285–7. https://doi.org/10.1016/j.leukres.2009.02.024.
Franceschino A, Tornaghi L, Benemacher V, Assouline S, Gambacorti-Passerini C. Alterations in creatine kinase, phosphate and lipid values in patients with chronic myeloid leukemia during treatment with imatinib. Haematologica. 2008;93(2):317–8. https://doi.org/10.3324/haematol.11680.
Gottardi M, Manzato E, Gherlinzoni F. Imatinib and hyperlipidemia. N Engl J Med. 2005;353(25):2722–3. https://doi.org/10.1056/NEJMc052500.
Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376(10):917–27. https://doi.org/10.1056/NEJMoa1609324.
Ellis M, Krashin E, Hamburger-Avnery O, Gan S, Elis A, Ashur-Fabian O. The anti-leukemic and lipid lowering effects of imatinib are not hindered by statins in CML: a retrospective clinical study and in vitro assessment of lipid-genes transcription. Leuk Lymphoma. 2017;58(5):1172–7. https://doi.org/10.1080/10428194.2016.1228928.
Klawitter J, Anderson N, Klawitter J, Christians U, Leibfritz D, Eckhardt SG, et al. Time-dependent effects of imatinib in human leukaemia cells: a kinetic NMR-profiling study. Br J Cancer. 2009;100(6):923–31. https://doi.org/10.1038/sj.bjc.6604946.
Li Z, Zhang H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol Life Sci. 2016;73(2):377–92. https://doi.org/10.1007/s00018-015-2070-4.
Wei Z, Liu X, Cheng C, Yu W, Yi P. Metabolism of amino acids in cancer. Front Cell Dev Biol. 2021. https://doi.org/10.3389/fcell.2020.603837.
Lieu EL, Nguyen T, Rhyne S, Kim J. Amino acids in cancer. Exp Mol Med. 2020;52(1):15–30. https://doi.org/10.1038/s12276-020-0375-3.
Amelio I, Cutruzzolá F, Antonov A, Agostini M, Melino G. Serine and glycine metabolism in cancer. Trends Biochem Sci. 2014;39(4):191–8. https://doi.org/10.1016/j.tibs.2014.02.004.
Kalhan SC, Hanson RW. Resurgence of serine: an often neglected but indispensable amino acid. J Biol Chem. 2012;287(24):19786–91. https://doi.org/10.1074/jbc.R112.357194.
Zhang B, Dong L-W, Tan Y-X, Zhang J, Pan Y-F, Yang C, et al. Asparagine synthetase is an independent predictor of surgical survival and a potential therapeutic target in hepatocellular carcinoma. Br J Cancer. 2013;109(1):14–23. https://doi.org/10.1038/bjc.2013.293.
Nagarajan A, Malvi P, Wajapeyee N. Oncogene-directed alterations in cancer cell metabolism. Trends Cancer. 2016;2(7):365–77. https://doi.org/10.1016/j.trecan.2016.06.002.
Dillon BJ, Prieto VG, Curley SA, Ensor CM, Holtsberg FW, Bomalaski JS, et al. Incidence and distribution of argininosuccinate synthetase deficiency in human cancers. Cancer. 2004;100(4):826–33. https://doi.org/10.1002/cncr.20057.
Cui H, Darmanin S, Natsuisaka M, Kondo T, Asaka M, Shindoh M, et al. Enhanced expression of asparagine synthetase under glucose-deprived conditions protects pancreatic cancer cells from apoptosis induced by glucose deprivation and cisplatin. Cancer Res. 2007;67(7):3345–55. https://doi.org/10.1158/0008-5472.CAN-06-2519.
Lorenzi PL, Llamas J, Gunsior M, Ozbun L, Reinhold WC, Varma S, et al. Asparagine synthetase is a predictive biomarker of l-asparaginase activity in ovarian cancer cell lines. Mol Cancer Ther. 2008;7(10):3123–8. https://doi.org/10.1158/1535-7163.MCT-08-0589.
Xu Y, Lv F, Zhu X, Wu Y, Shen X. Loss of asparagine synthetase suppresses the growth of human lung cancer cells by arresting cell cycle at G0/G1 phase. Cancer Gene Ther. 2016;23(9):287–94. https://doi.org/10.1038/cgt.2016.28.
Yu Q, Wang X, Wang L, Zheng J, Wang J, Wang B. Knockdown of asparagine synthetase (ASNS) suppresses cell proliferation and inhibits tumor growth in gastric cancer cells. Scand J Gastroenterol. 2016;51(10):1220–6. https://doi.org/10.1080/00365521.2016.1190399.
Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9(10):1269–74. https://doi.org/10.1038/nm934.
Poliaková M, Aebersold DM, Zimmer Y, Medová M. The relevance of tyrosine kinase inhibitors for global metabolic pathways in cancer. Mol Cancer. 2018;17(1):1–12. https://doi.org/10.1186/s12943-018-0798-9.
Taymaz-Nikerel H, Eraslan S, Kırdar B. Insights into the mechanism of anticancer drug imatinib revealed through multi-omic analyses in yeast. OMICS. 2020;24(11):667–78. https://doi.org/10.1089/omi.2020.0144.
dos Santos SC, Mira NP, Moreira AS, Sá-Correia I. Quantitative- and phospho-proteomic analysis of the yeast response to the tyrosine kinase inhibitor imatinib to pharmacoproteomics-guided drug line extension. OMICS. 2012;16(10):537–51. https://doi.org/10.1089/omi.2012.0012.
Smith AM, Ammar R, Nislow C, Giaever G. A survey of yeast genomic assays for drug and target discovery. Pharmacol Ther. 2010;127(2):156–64. https://doi.org/10.1016/j.pharmthera.2010.04.012.
Guaragnella N, Palermo V, Galli A, Moro L, Mazzoni C, Giannattasio S. The expanding role of yeast in cancer research and diagnosis: insights into the function of the oncosuppressors p53 and BRCA1/2. FEMS Yeast Res. 2014;14(1):2–16. https://doi.org/10.1111/1567-1364.12094.
Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830(5):3143–53. https://doi.org/10.1016/j.bbagen.2012.09.008.
Cox AG, Hwang KL, Brown KK, Evason KJ, Beltz S, Tsomides A, et al. Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth. Nat Cell Biol. 2016;18(8):886–96. https://doi.org/10.1038/ncb3389.
West TP. Pyrimidine nucleotide synthesis in Pseudomonas nitroreducens and the regulatory role of pyrimidines. Microbiol Res. 2014;169(12):954–8. https://doi.org/10.1016/j.micres.2014.04.003.
Tibbetts AS, Appling DR. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30:57–81. https://doi.org/10.1146/annurev.nutr.012809.104810.
Gerber G, Siems W, Werner A, Dubiel W, Grune T, Henke W, et al. Dynamics in the purine nucleotides of liver during various periods of hypoxia/ischaemia and reoxygenation. Boston: Springer; 1991. p. 259–64.
Miller SG, Hafen PS, Brault JJ. Increased adenine nucleotide degradation in skeletal muscle atrophy. Int J Mol Sci. 2019;21(1):88. https://doi.org/10.3390/ijms21010088.
Ma J, Zhong M, Xiong Y, Gao Z, Wu Z, Liu Y, et al. Emerging roles of nucleotide metabolism in cancer development: progress and prospect. Aging. 2021;13(9):13349–58. https://doi.org/10.18632/aging.202962.
Madaan K, Kaushik D, Verma T. Hydroxyurea: a key player in cancer chemotherapy. Expert Rev Anticancer Ther. 2012;12(1):19–29. https://doi.org/10.1586/era.11.175.
Szekeres T, Gharehbaghi K, Fritzer M, Woody M, Srivastava A, v’ant Riet B, Jayaram HN, Elford HL. Biochemical and antitumor activity of trimidox, a new inhibitor of ribonucleotide reductase. Cancer Chemother Pharmacol. 1994;34(1):63–6. https://doi.org/10.1007/BF00686113.
Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96(3):925–32.
Nowicki MO, Pawlowski P, Fischer T, Hess G, Pawlowski T, Skorski T. Chronic myelogenous leukemia molecular signature. Oncogene. 2003;22(25):3952–63. https://doi.org/10.1038/sj.onc.1206620.
Gu JJ, Santiago L, Mitchell BS. Synergy between imatinib and mycophenolic acid in inducing apoptosis in cell lines expressing Bcr-Abl. Blood. 2005;105(8):3270–7. https://doi.org/10.1182/blood-2004-10-3864.
Kroschwald L, Suttorp M, Tauer J, Zimmermann N, Gunther C, Bauer A. Off-target effect of imatinib and nilotinib on human vitamin D3 metabolism. Mol Med Rep. 2017;17(1):1382–8. https://doi.org/10.3892/mmr.2017.7952.
Damaraju VL, Kuzma M, Cass CE, Putman CT, Sawyer MB. Multitargeted kinase inhibitors imatinib, sorafenib and sunitinib perturb energy metabolism and cause cytotoxicity to cultured C2C12 skeletal muscle derived myotubes. Biochem Pharmacol. 2018;155:162–71. https://doi.org/10.1016/j.bcp.2018.07.001.
Bouitbir J, Panajatovic MV, Frechard T, Roos NJ, Krähenbühl S. Imatinib and dasatinib provoke mitochondrial dysfunction leading to oxidative stress in C2C12 myotubes and human RD cells. Front Pharmacol. 2020. https://doi.org/10.3389/fphar.2020.01106.
Fröbom R, Berglund E, Aspinwall CA, Lui W-O, Nilsson I-L, Larsson C, et al. Direct interaction of the ATP-sensitive K+ channel by the tyrosine kinase inhibitors imatinib, sunitinib and nilotinib. Biochem Biophys Res Commun. 2021;557:14–9. https://doi.org/10.1016/j.bbrc.2021.03.166.
Acknowledgements
The authors acknowledge the support received from the Banaras Hindu University, Varanasi in writing this manuscript. VK, PS, and VA are grateful to the CSIR, New Delhi, to award their fellowships.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
MV conceived and designed this review. VK collected the literature and prepared the first draft of the manuscript. PS edited the manuscript and generated the figures. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any authors.
Consent to participate
This article does not contain any studies with human participants.
Consent to publish
This article does not contain any studies with human research participants, so informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumar, V., Singh, P., Gupta, S.K. et al. Alterations in cellular metabolisms after Imatinib therapy: a review. Med Oncol 39, 95 (2022). https://doi.org/10.1007/s12032-022-01699-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01699-8