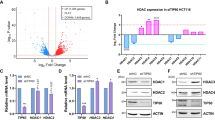
Abstract
It has been recently revealed that Histone Deacetylase (HDAC) 3, a unique member of the HDACs family, can trigger and progress cancers by alternation in genes expression and proteins activity. Epigenetic modifications by HDACs have been studied well in various cancer cells. Recent studies have focused on the HDAC enzymes as a possible target in cancer therapy. There are significant documents on upregulation of HDAC3 in breast cancer (BC) cells which suggest an oncogenic role for this enzyme. Interestingly, some studies showed that HDAC3 inhibition could be considered as a promising target in breast cancer therapy, and thus far, several inhibitors from different nature have been introduced. In this review, we discussed the function and highlight the existing inhibitors of HDAC3 in BC pathogenesis and therapy.



Similar content being viewed by others
Abbreviations
- APL:
-
Acute promyelocytic leukemia
- AR:
-
Androgen receptor
- BC:
-
Breast cancer
- Bcl6:
-
B-cell lymphoma 6
- CK2:
-
Casein kinase
- c-Myc:
-
Cellular myelocytomatosis
- CSC:
-
Cancer stem cell
- E2F1:
-
Transcription factor E2F1
- EGF:
-
Epidermal growth factor
- EGFR:
-
Epidermal growth factor receptor
- EMT:
-
Epithelial mesenchymal transition
- ER:
-
Estrogen receptor
- G2:
-
Growth 2 phase
- GATA1:
-
GATA-binding factors
- GSK:
-
Glycogen Synthase Kinase
- HATs:
-
Histone acetyltransferases
- HDAC3:
-
Histone deacetylase-3
- HDACi:
-
Histone deacetylase inhibitor
- HDACs:
-
Histone deacetylases
- HER2:
-
Human epidermal growth factor receptor 2
- HIF-1 α:
-
Hypoxia-inducible factor 1α
- HIPK2:
-
Homeodomain-interacting protein kinase 2
- HCC:
-
Hepato-cellular carcinoma
- HE4:
-
Human epididymis protein 4
- Hsp90:
-
Heat shock protein 90
- IAPs:
-
Inhibitor of apoptosis proteins
- JNK:
-
Jun N-terminal kinase
- KDELR2:
-
KDEL (Lys-Asp-Glu-Leu) receptor 2
- M:
-
Mitosis phase
- MAPK:
-
Mitogen-activated protein kinase
- MiRNAs:
-
MicroRNAs
- Mi-2/NuRD:
-
Nucleosome remodeling deacetylase
- MM:
-
Multiple myeloma
- MyoD:
-
Myogenic regulatory factor
- NAD+ :
-
Nicotinamide adenine dinucleotide
- NF-kB:
-
Nuclear factor κB
- N-CoR:
-
Nuclear receptor co-repressor
- P21:
-
Potent cyclin-dependent kinase inhibitor
- p300:
-
Histone acetyltransferase belongs to the p300/CBP family
- p53:
-
Tumor protein 53
- PD-L1:
-
Programmed death ligand 1
- PI3Ks:
-
Phosphoinositide 3-kinase
- POC5:
-
Protein Coding5
- PR:
-
Progesterone receptor
- PUMA:
-
P53-upregulated modulator of apoptosis
- ROS:
-
Reactive oxygen species
- RUNEX3:
-
Core binding factor α3 subunit
- SAHA:
-
Suberanilohydroxamic acid
- SENP1:
-
Sentrin-specific protease 1
- Sin3:
-
Swi independent
- siRNAs:
-
Small interfering RNAs
- SIRT:
-
NAD-dependent deacetylase sirtuin
- SMRTs:
-
Silencing mediator of retinoid and thyroid-hormone receptors
- STAT3:
-
Signal transduction and activation of transcription 3
- YY1:
-
Transcription factor Yin Yang 1
- TFIIE:
-
Transcription factor II E
- TFIIF:
-
Transcription factor II F
- TNBC:
-
Triple-negative breast cancer
- TNF-α:
-
Tumor necrosis factor-α
- TRAIL:
-
Tumor necrosis factor-related apoptosis-inducing ligand
- XIAP:
-
X-linked inhibitor of apoptosis protein
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Lukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanislawek A. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers (Basel). 2021;13(17):4287.
Tan PH, Ellis I, Allison K, Brogi E, Fox SB, Lakhani S, et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology. 2020;77(2):181–5.
Byler S, Goldgar S, Heerboth S, Leary M, Housman G, Moulton K, et al. Genetic and epigenetic aspects of breast cancer progression and therapy. Anticancer Res. 2014;34(3):1071–7.
Karsli-Ceppioglu S, Dagdemir A, Judes G, Ngollo M, Penault-Llorca F, Pajon A, et al. Epigenetic mechanisms of breast cancer: an update of the current knowledge. Epigenomics. 2014;6(6):651–64.
Momenimovahed Z, Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer (Dove Med Press). 2019;11:151–64.
Biswas S, Rao CM. Epigenetics in cancer: fundamentals and beyond. Pharmacol Ther. 2017;173:118–34.
Perri F, Longo F, Giuliano M, Sabbatino F, Favia G, Ionna F, et al. Epigenetic control of gene expression: potential implications for cancer treatment. Crit Rev Oncol Hematol. 2017;111:166–72.
Eckschlager T, Plch J, Stiborova M, Hrabeta J. Histone deacetylase inhibitors as anticancer drugs. Int J Mol Sci. 2017;18(7):1414.
Baylin SB, Jones PA. Epigenetic determinants of cancer. Cold Spring Harb Perspect Biol. 2016;8(9):a019505.
Li G, Tian Y, Zhu WG. The roles of histone deacetylases and their inhibitors in cancer therapy. Front Cell Dev Biol. 2020;8:576946.
Mottamal M, Zheng S, Huang TL, Wang G. Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules. 2015;20(3):3898–941.
Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol. 2014;6(4):a018713.
Patra S, Panigrahi DP, Praharaj PP, Bhol CS, Mahapatra KK, Mishra SR, et al. Dysregulation of histone deacetylases in carcinogenesis and tumor progression: a possible link to apoptosis and autophagy. Cell Mol Life Sci. 2019;76(17):3263–82.
Barneda-Zahonero B, Parra M. Histone deacetylases and cancer. Mol Oncol. 2012;6(6):579–89.
Muller BM, Jana L, Kasajima A, Lehmann A, Prinzler J, Budczies J, et al. Differential expression of histone deacetylases HDAC1, 2 and 3 in human breast cancer–overexpression of HDAC2 and HDAC3 is associated with clinicopathological indicators of disease progression. BMC Cancer. 2013;13:215.
Salek Farrokhi A, Mohammadlou M, Abdollahi M, Eslami M, Yousefi B. Histone deacetylase modifications by probiotics in colorectal cancer. J Gastrointest Cancer. 2020;51(3):754–64.
Moufarrij S, Dandapani M, Arthofer E, Gomez S, Srivastava A, Lopez-Acevedo M, et al. Epigenetic therapy for ovarian cancer: promise and progress. Clin Epigenetics. 2019;11(1):7.
Tchio Mantho CI, Harbuzariu A, Gonzalez-Perez RR. Histone deacetylases, microRNA and leptin crosstalk in pancreatic cancer. World J Clin Oncol. 2017;8(3):178–89.
Darvishi N, Rahimi K, Mansouri K, Fathi F, Menbari MN, Mohammadi G, et al. MiR-646 prevents proliferation and progression of human breast cancer cell lines by suppressing HDAC2 expression. Mol Cell Probes. 2020;53:101649.
Menbari MN, Rahimi K, Ahmadi A, Elyasi A, Darvishi N, Hosseini V, et al. MiR-216b-5p inhibits cell proliferation in human breast cancer by down-regulating HDAC8 expression. Life Sci. 2019;237:116945.
Menbari MN, Rahimi K, Ahmadi A, Mohammadi-Yegane S, Elyasi A, Darvishi N, et al. Association of HDAC8 expression with pathological findings in triple negative and non-triple negative breast cancer: implications for diagnosis. Iran Biomed J. 2020;24(5):288–94.
Menbari MN, Rahimi K, Ahmadi A, Mohammadi-Yeganeh S, Elyasi A, Darvishi N, et al. miR-483-3p suppresses the proliferation and progression of human triple negative breast cancer cells by targeting the HDAC8>oncogene. J Cell Physiol. 2020;235(3):2631–42.
Rahmani G, Sameri S, Abbasi N, Abdi M, Najafi R. The clinical significance of histone deacetylase-8 in human breast cancer. Pathol Res Pract. 2021;220:153396.
Ramaiah MJ, Tangutur AD, Manyam RR. Epigenetic modulation and understanding of HDAC inhibitors in cancer therapy. Life Sci. 2021;277:119504.
Adhikari N, Amin SA, Trivedi P, Jha T, Ghosh B. HDAC3 is a potential validated target for cancer: an overview on the benzamide-based selective HDAC3 inhibitors through comparative SAR/QSAR/QAAR approaches. Eur J Med Chem. 2018;157:1127–42.
Zhang L, Chen Y, Jiang Q, Song W, Zhang L. Therapeutic potential of selective histone deacetylase 3 inhibition. Eur J Med Chem. 2019;162:534–42.
Sarkar R, Banerjee S, Amin SA, Adhikari N, Jha T. Histone deacetylase 3 (HDAC3) inhibitors as anticancer agents: a review. Eur J Med Chem. 2020;192:112171.
Ishii S. The role of histone deacetylase 3 complex in nuclear hormone receptor action. Int J Mol Sci. 2021;22(17):9138.
Watson PJ, Fairall L, Santos GM, Schwabe JW. Structure of HDAC3 bound to co-repressor and inositol tetraphosphate. Nature. 2012;481(7381):335–40.
Liang N, Jakobsson T, Fan R, Treuter E. The nuclear receptor-co-repressor complex in control of liver metabolism and disease. Front Endocrinol (Lausanne). 2019;10:411.
Kwak SM, Seo J, Hwang JT, Sung GJ, Song JH, Jeong JH, et al. EGFR-c-Src-mediated HDAC3 phosphorylation exacerbates invasion of breast cancer cells. Cells. 2019;8(8):930.
Kwon Y, Kim Y, Jung HS, Jeoung D. Role of HDAC3-miRNA-CAGE network in anti-cancer drug-resistance. Int J Mol Sci. 2018;20(1):51.
Seo J, Guk G, Park SH, Jeong MH, Jeong JH, Yoon HG, et al. Tyrosine phosphorylation of HDAC3 by Src kinase mediates proliferation of HER2-positive breast cancer cells. J Cell Physiol. 2019;234(5):6428–36.
Zhang F, Qi L, Feng Q, Zhang B, Li X, Liu C, et al. HIPK2 phosphorylates HDAC3 for NF-kappaB acetylation to ameliorate colitis-associated colorectal carcinoma and sepsis. Proc Natl Acad Sci U S A. 2021;118(28):e2021798118.
Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26(37):5420–32.
Lkhagva B, Kao YH, Lee TI, Lee TW, Cheng WL, Chen YJ. Activation of class I histone deacetylases contributes to mitochondrial dysfunction in cardiomyocytes with altered complex activities. Epigenetics. 2018;13(4):376–85.
Xu Z, Tong Q, Zhang Z, Wang S, Zheng Y, Liu Q, et al. Inhibition of HDAC3 prevents diabetic cardiomyopathy in OVE26 mice via epigenetic regulation of DUSP5-ERK1/2 pathway. Clin Sci (Lond). 2017;131(15):1841–57.
He X, Zhang L, Queme LF, Liu X, Lu A, Waclaw RR, et al. A histone deacetylase 3-dependent pathway delimits peripheral myelin growth and functional regeneration. Nat Med. 2018;24(3):338–51.
Siebzehnrubl FA, Raber KA, Urbach YK, Schulze-Krebs A, Canneva F, Moceri S, et al. Early postnatal behavioral, cellular, and molecular changes in models of Huntington disease are reversible by HDAC inhibition. Proc Natl Acad Sci U S A. 2018;115(37):E8765–74.
Amin SA, Adhikari N, Kotagiri S, Jha T, Ghosh B. Histone deacetylase 3 inhibitors in learning and memory processes with special emphasis on benzamides. Eur J Med Chem. 2019;166:369–80.
Zhao B, Yuan Q, Hou JB, Xia ZY, Zhan LY, Li M, et al. Inhibition of HDAC3 ameliorates cerebral ischemia reperfusion injury in diabetic mice in vivo and in vitro. J Diabetes Res. 2019;2019:8520856.
Tong L, Liang H, Zhuang H, Liu C, Zhang Z. The relationship between HDAC3 and malignant tumors: a mini review. Crit Rev Eukaryot Gene Expr. 2020;30(3):279–84.
Adhikari N, Jha T, Ghosh B. Dissecting histone deacetylase 3 in multiple disease conditions: selective inhibition as a promising therapeutic strategy. J Med Chem. 2021;64(13):8827–69.
Ma L, Qi L, Li S, Yin Q, Liu J, Wang J, et al. Aberrant HDAC3 expression correlates with brain metastasis in breast cancer patients. Thorac Cancer. 2020;11(9):2493–505.
Weichert W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009;280(2):168–76.
Xu G, Zhu H, Zhang M, Xu J. Histone deacetylase 3 is associated with gastric cancer cell growth via the miR-454-mediated targeting of CHD5. Int J Mol Med. 2018;41(1):155–63.
Yang Z, Jiang X, Zhang Z, Zhao Z, Xing W, Liu Y, et al. HDAC3-dependent transcriptional repression of FOXA2 regulates FTO/m6A/MYC signaling to contribute to the development of gastric cancer. Cancer Gene Ther. 2021;28(1–2):141–55.
Zhang L, Liu F, Meng Z, Luo Q, Pan D, Qian Y. Inhibited HDAC3 promotes microRNA-376c-3p to suppress malignant phenotypes of gastric cancer cells by reducing WNT2b. Genomics. 2021;113(6):3512–22.
Wu SM, Jan YJ, Tsai SC, Pan HC, Shen CC, Yang CN, et al. Targeting histone deacetylase-3 blocked epithelial-mesenchymal plasticity and metastatic dissemination in gastric cancer. Cell Biol Toxicol. 2022. https://doi.org/10.1007/s10565-021-09673-2.
Li H, Li H, Waresijiang Y, Chen Y, Li Y, Yu L, et al. Clinical significance of HDAC1, -2 and -3 expression levels in esophageal squamous cell carcinoma. Exp Ther Med. 2020;20(1):315–24.
Lucio-Eterovic AK, Cortez MA, Valera ET, Motta FJ, Queiroz RG, Machado HR, et al. Differential expression of 12 histone deacetylase (HDAC) genes in astrocytomas and normal brain tissue: class II and IV are hypoexpressed in glioblastomas. BMC Cancer. 2008;8:243.
Nemati M, Ajami N, Estiar MA, Rezapour S, Gavgani RR, Hashemzadeh S, et al. Deregulated expression of HDAC3 in colorectal cancer and its clinical significance. Adv Clin Exp Med. 2018;27(3):305–11.
Ren H, Tang L. HDAC3-mediated lncRNA-LOC101928316 contributes to cisplatin resistance in gastric cancer via activating the PI3K-Akt-mTOR pathway. Neoplasma. 2021;68(5):1043–51.
Zhang L, Hong Z, Zhang RR, Sun XZ, Yuan YF, Hu J, et al. Bakkenolide A inhibits leukemia by regulation of HDAC3 and PI3K/Akt-related signaling pathways. Biomed Pharmacother. 2016;83:958–66.
Lou T, Zhuang H, Liu C, Zhang Z. HDAC3 positively regulates HE4 expression to promote ovarian carcinoma progression. Arch Biochem Biophys. 2019;675:108044.
Quiroz-Reyes AG, Delgado-Gonzalez P, Islas JF, Gallegos JLD, Martinez Garza JH, Garza-Trevino EN. Behind the adaptive and resistance mechanisms of cancer stem cells to TRAIL. Pharmaceutics. 2021;13(7):1062.
Zhang B, Liu B, Chen D, Setroikromo R, Haisma HJ, Quax WJ. Histone deacetylase inhibitors sensitize TRAIL-induced apoptosis in colon cancer cells. Cancers (Basel). 2019;11(5):645.
Lombard DB, Cierpicki T, Grembecka J. Combined MAPK pathway and HDAC inhibition breaks melanoma. Cancer Discov. 2019;9(4):469–71.
Trivedi P, Adhikari N, Amin SA, Jha T, Ghosh B. Design, synthesis and biological screening of 2-aminobenzamides as selective HDAC3 inhibitors with promising anticancer effects. Eur J Pharm Sci. 2018;124:165–81.
Zhou L, Xu X, Liu H, Hu X, Zhang W, Ye M, et al. Prognosis analysis of histone deacetylases mRNA expression in ovarian cancer patients. J Cancer. 2018;9(23):4547–55.
Hu G, He N, Cai C, Cai F, Fan P, Zheng Z, et al. HDAC3 modulates cancer immunity via increasing PD-L1 expression in pancreatic cancer. Pancreatology. 2019;19(2):383–9.
Shouksmith AE, Shah F, Grimard ML, Gawel JM, Raouf YS, Geletu M, et al. Identification and characterization of AES-135, a hydroxamic acid-based HDAC inhibitor that prolongs survival in an orthotopic mouse model of pancreatic cancer. J Med Chem. 2019;62(5):2651–65.
Cui Z, Xie M, Wu Z, Shi Y. Relationship between histone deacetylase 3 (HDAC3) and breast cancer. Med Sci Monit. 2018;24:2456–64.
Mirsadeghi L, Haji Hosseini R, Banaei-Moghaddam AM, Kavousi K. EARN: an ensemble machine learning algorithm to predict driver genes in metastatic breast cancer. BMC Med Genomics. 2021;14(1):122.
Su W, Zeng L, Chen W. Moscatilin suppresses the breast cancer both in vitro and in vivo by inhibiting HDAC3. Dose Response. 2021;19(1):15593258211001252.
Yang M, Dang X, Tan Y, Wang M, Li X, Li G. I-7ab inhibited the growth of TNBC cells via targeting HDAC3 and promoting the acetylation of p53. Biomed Pharmacother. 2018;99:220–6.
Chen SY, Teng SC, Cheng TH, Wu KJ. miR-1236 regulates hypoxia-induced epithelial-mesenchymal transition and cell migration/invasion through repressing SENP1 and HDAC3. Cancer Lett. 2016;378(1):59–67.
Ververis K, Karagiannis TC. An atlas of histone deacetylase expression in breast cancer: fluorescence methodology for comparative semi-quantitative analysis. Am J Transl Res. 2012;4(1):24–43.
Bayat S, Mansoori Derakhshan S, Mansoori Derakhshan N, Shekari Khaniani M, Alivand MR. Downregulation of HDAC2 and HDAC3 via oleuropein as a potent prevention and therapeutic agent in MCF-7 breast cancer cells. J Cell Biochem. 2019;120(6):9172–80.
Lee JY, Kuo CW, Tsai SL, Cheng SM, Chen SH, Chan HH, et al. Inhibition of HDAC3- and HDAC6-promoted survivin expression plays an important role in SAHA-induced autophagy and viability reduction in breast cancer cells. Front Pharmacol. 2016;7:81.
Hanigan TW, Aboukhatwa SM, Taha TY, Frasor J, Petukhov PA. Divergent JNK phosphorylation of HDAC3 in triple-negative breast cancer cells determines HDAC inhibitor binding and selectivity. Cell Chem Biol. 2017;24(11):1356-67 e8.
Qiao W, Liu H, Liu R, Liu Q, Zhang T, Guo W, et al. Prognostic and clinical significance of histone deacetylase 1 expression in breast cancer: a meta-analysis. Clin Chim Acta. 2018;483:209–15.
Wei H, Ma W, Lu X, Liu H, Lin K, Wang Y, et al. KDELR2 promotes breast cancer proliferation via HDAC3-mediated cell cycle progression. Cancer Commun (London). 2021. https://doi.org/10.1002/cac2.12180.
Krusche CA, Wulfing P, Kersting C, Vloet A, Bocker W, Kiesel L, et al. Histone deacetylase-1 and -3 protein expression in human breast cancer: a tissue microarray analysis. Breast Cancer Res Treat. 2005;90(1):15–23.
Seo J, Min SK, Park HR, Kim DH, Kwon MJ, Kim LS, et al. Expression of histone deacetylases HDAC1, HDAC2, HDAC3, and HDAC6 in invasive ductal carcinomas of the breast. J Breast Cancer. 2014;17(4):323–31.
Park SH, Kim H, Kwak S, Jeong JH, Lee J, Hwang JT, et al. HDAC3-ERalpha selectively regulates TNF-alpha-induced apoptotic cell death in MCF-7 human breast cancer cells via the p53 signaling pathway. Cells. 2020;9(5):1280.
Oie S, Matsuzaki K, Yokoyama W, Murayama A, Yanagisawa J. HDAC3 regulates stability of estrogen receptor alpha mRNA. Biochem Biophys Res Commun. 2013;432(2):236–41.
Yu S, Gong X, Ma Z, Zhang M, Huang L, Zhang J, et al. Endocrine resistant breast cancer cells with loss of ERalpha expression retain proliferative ability by reducing caspase7-mediated HDAC3 cleavage. Cell Oncol (Dordr). 2020;43(1):65–80.
Zhao Y, He J, Yang L, Luo Q, Liu Z. Histone deacetylase-3 modification of MicroRNA-31 promotes cell proliferation and aerobic glycolysis in breast cancer and is predictive of poor prognosis. J Breast Cancer. 2018;21(2):112–23.
Feng W, Lu Z, Luo RZ, Zhang X, Seto E, Liao WS, et al. Multiple histone deacetylases repress tumor suppressor gene ARHI in breast cancer. Int J Cancer. 2007;120(8):1664–8.
Darvin P, Sasidharan Nair V, Elkord E. PD-L1 expression in human breast cancer stem cells is epigenetically regulated through posttranslational histone modifications. J Oncol. 2019;2019:3958908.
Fermento ME, Gandini NA, Salomon DG, Ferronato MJ, Vitale CA, Arevalo J, et al. Inhibition of p300 suppresses growth of breast cancer. Role of p300 subcellular localization. Exp Mol Pathol. 2014;97(3):411–24.
Lucas J, Hsieh TC, Halicka HD, Darzynkiewicz Z, Wu JM. Upregulation of PDL1 expression by resveratrol and piceatannol in breast and colorectal cancer cells occurs via HDAC3/p300mediated NFkappaB signaling. Int J Oncol. 2018;53(4):1469–80.
Liu J, He D, Cheng L, Huang C, Zhang Y, Rao X, et al. p300/CBP inhibition enhances the efficacy of programmed death-ligand 1 blockade treatment in prostate cancer. Oncogene. 2020;39(19):3939–51.
Hsieh HY, Chuang HC, Shen FH, Detroja K, Hsin LW, Chen CS. Targeting breast cancer stem cells by novel HDAC3-selective inhibitors. Eur J Med Chem. 2017;140:42–51.
Routholla G, Pulya S, Patel T, Abdul Amin S, Adhikari N, Biswas S, et al. Synthesis, biological evaluation, and molecular docking analysis of novel linker-less benzamide based potent and selective HDAC3 inhibitors. Bioorgan Chem. 2021;114:105050.
Putri AD, Chen PS, Su YL, Lin JP, Liou JP, Hsieh CM. Optimization and development of selective histone deacetylase inhibitor (MPT0B291)-loaded albumin nanoparticles for anticancer therapy. Pharmaceutics. 2021;13(10):1728.
Luo G, Lin X, Ren S, Wu S, Wang X, Ma L, et al. Development of novel tetrahydroisoquinoline-hydroxamate conjugates as potent dual SERDs/HDAC inhibitors for the treatment of breast cancer. Eur J Med Chem. 2021;226:113870.
Subramanian S, Bates SE, Wright JJ, Espinoza-Delgado I, Piekarz RL. Clinical toxicities of histone deacetylase inhibitors. Pharmaceuticals (Basel). 2010;3(9):2751–67.
Benedetti R, Conte M, Altucci L. Targeting histone deacetylases in diseases: where are we? Antioxid Redox Signal. 2015;23(1):99–126.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
MA conceived the essay; RR researched the literature and wrote the manuscript; MA, YR, and MHK-A revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no financial relationships relevant to this article to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahbari, R., Rasmi, Y., Khadem-Ansari, M.H. et al. The role of histone deacetylase 3 in breast cancer. Med Oncol 39, 84 (2022). https://doi.org/10.1007/s12032-022-01681-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01681-4