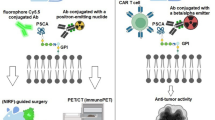
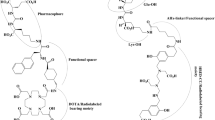
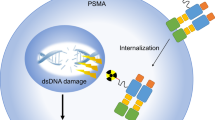
Abstract
Although management of advanced prostate cancer is evolving, a lot of work remains to be done for patients who have exhausted all options. Molecular targeting of prostate specific membrane antigen (PSMA) is valuable not only for diagnostic but also for therapeutic reasons. PSMA is thus considered to be useful in a theranostic approach. PSMA scans are upcoming diagnostic modalities which detect metastatic lesions that are missed by conventional imaging modalities. PSMA ligand therapy is also an upcoming treatment modality that has been proven to be beneficial with minimal toxicity in patients with advanced prostate cancer that have progressed on prior therapy. In this review article, we summarize the current knowledge regarding PSMA diagnostics and PSMA ligand therapies and discuss their implication in the treatment of advanced prostate cancer.








Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
Parker C, Gillessen S, Heidenreich A, Horwich A, Committee EG. Cancer of the prostate:ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(5):v69–77.
Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987;7:927–35.
Kawakami M, Nakayama J. Enhanced expression of prostate-specific membrane antigen gene in prostate cancer as revealed by in situ hybridization. Cancer Res. 1997;57:2321–4.
Silver DA, Pellicer I, Fair WR, et al. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–5.
Diane A et al. Prostate cancer theranostics-an overview. Front Oncol. 2020. https://doi.org/10.3389/fonc.2020.00884.
Wright GL Jr, et al. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol Semin Origin Investig. 1995;1(1):18–28.
Bostwick DG, et al. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82(11):2256–61.
Kusumi T, et al. Immunohistochemical detection of carcinoma in radical prostatectomy specimens following hormone therapy. Pathol Int. 2008;58(11):687–94.
Mannweiler S, et al. Heterogeneity of prostate-specific membrane antigen (psma) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res. 2009;15(2):167–72.
Bouchelouche K, Choyke PL, Capala J. Prostate specific membrane antigen—A target for imaging and therapy with radionuclides. Discov Med. 2010;9:55–61.
Backhaus P, Noto B, Avramovic N, et al. Targeting PSMA by radioligands in non-prostate disease-current status and future perspectives. Eur J Nucl Med Mol Imaging. 2018;45:860–77.
Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted α-therapy of metastatic castrationresistant prostate cancer with 225Ac-PSMA-617: dosimetry estimate and empiric dose finding. J Nucl Med. 2017;58:1624–31.
Pinto JT, et al. Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells. Clin Cancer Res. 1996;2(9):1445–51.
Carter RE, Feldman AR, Coyle JT. Prostate-specific membrane antigen Is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc Natl Acad Sci USA. 1996;93(2):749–53.
Halsted CH, et al. Folylpoly-γ-glutamate carboxypeptidase from Pig jejunum. molecular characterization and relation to glutamate carboxypeptidase Ii. J Biol Chem. 1998;273(32):20417–24.
Rajasekaran SA, et al. A novel cytoplasmic tail MXXXL motif mediates the internalization of prostate-specific membrane antigen. Mol Biol Cell. 2003;14(12):4835–45.
Ruigrok EAM, van Weerden WM, Nonnekens J, et al. The future of PSMA-targeted radionuclide therapy: an overview of recent preclinical research. Pharmaceutics. 2019;11(11):560.
Rahbar K, Afshar-Oromieh A, Jadvar H, et al. PSMA theranostics: current status and future directions. Mol Imaging. 2018;17:1536012118776068.
Kopka K, Benesova M, Barinka C, et al. Glu-ureido-based inhibitors of prostate-specific membrane antigen: lessons learned during the development of a novel class of low-molecular weight theranostic radiotracers. J Nucl Med. 2017;58(2):17S-26S.
Wang Y, Wang S, Huang M. Structure and enzymatic activities of human serum albumin. Curr Pharm Des. 2015;21:1831–6.
Infusino I, Panteghini M. Serum albumin: accuracy and clinical use. Clin Chim Acta. 2013;419:15–8.
Wang Z, Jacobson O, Tian R, et al. Radioligand therapy of prostate cancer with a long-lasting prostate-specific membrane antigen targeting agent 90Y-DOTA-EB-MCG. Bioconjug Chem. 2018;29:2309–15.
Merlot AM, Kalinowski DS, Richardson DR. Unraveling the mysteries of serum albumin-more than just a serum protein. Front Physiol. 2014;5:299.
Czerwińska M, Bilewicz A, Kruszewski M, et al. Targeted radionuclide therapy of prostate cancer—from basic research to clinical perspectives. Molecules. 2020;25(7):1743.
Benesova M, Bauder-Wust U, Schafer M, et al. Linker modification strategies to control. The prostate-specific membrane antigen (PSMA)-targeting and pharmacokinetic properties of DOTA conjugated PSMA inhibitors. J Med Chem. 2016;59:1761–75.
Wustemann T, Bauder-Wust U, Schafer M, et al. Design of internalizing PSMA-specific gluureido-based radiotherapeuticals. Theranostics. 2016;6:1085–95.
Haberkorn U, Eder M, Kopka K, et al. New strategies in prostate cancer: prostate-specific membrane antigen (PSMA) ligands for diagnosis and therapy. Clin Cancer Res. 2016;22(1):9–15.
Ray BS, Chen Z, Pullambhatla M, et al. Preclinical comparative study of 68Ga-labeled DOTA, NOTA, and HBED-CC chelated Radiotracers for targeting PSMA. Bioconjug Chem. 2016;27:1447–55.
Benesova M, Schafer M, Bauder-Wust U, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med. 2015;56:914–20.
Schmidt A, Wirtz M, Färber SF, et al. Effect of carbohydration on the theranostic tracer PSMA I&T. ACS Omega. 2018;3(7):8278–87.
Kuo HT, Pan J, Zhang Z, et al. Effects of linker modification on tumor-to-kidney contrast of 68Ga-labeled PSMA-targeted imaging probes. Mol Pharm. 2018;15:3502–11.
Kelly J, Amor-Coarasa A, Ponnala S, et al. Trifunctional PSMA-targeting constructs for prostate cancer with unprecedented localization to LNCaP tumors. Eur J Nucl Med Mol Imaging. 2018;45:1841–51.
Wurzer A, Seidl C, Morgenstern A, et al. Dual-nuclide Radiopharmaceuticals for positron emission tomography based dosimetry in radiotherapy. Chemistry. 2018;24:547–50.
Kassis AI. Therapeutic radionuclides: Biophysical and radiobiologic principles. Semin Nucl Med. 2008;38:358–66.
Cimadamore A, Cheng M, Santoni, , et al. New prostate cancer targets for diagnosis, imaging, and therapy: focus on prostate-specific membrane antigen. Front Oncol. 2018. https://doi.org/10.3389/fonc.2018.00653.
Rahbar K, Ahmadzadehfar H, Kratochwil C, et al. German Multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90.
Kratochwil C, Giesel FL, Stefanova M, et al. PSMA-Targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J Nucl Med. 2016;57:1170–6.
Champion C, Quinto MA, Morgat C, et al. Comparison between three promising ß-emitting radionuclides, 67Cu, 47Sc and 161Tb, with emphasis on doses delivered to minimal residual disease. Theranostics. 2016;6:1611–8.
Nonnekens J, Chatalic KL, Molkenboer-Kuenen JD, et al. 213Bi-labeled prostate-specific membrane antigen-targeting agents induce DNA double-strand breaks in prostate cancer xenografts. Cancer Biother Radiopharm. 2017;32:67–73.
Eppard E. Pre-therapeutic dosimetry employing scandium-44 for radiolabeling PSMA-617. In: Prostatectomy. https://www.intechopen.com/books/prostatectomy/pre-therapeuticdosimetry-employing-scandium-44-for-radiolabeling-psma-617. Accessed 10 Apr 2021.
Zustovich F, Barsanti R. Targeted α therapies for the treatment of bone metastases. Int J Mol Sci. 2017;19:74.
Kiess AP, Minn I, Vaidyanathan G, et al. (2S)-2-(3-(1-carboxy-5-(4–211At-Astatobenzamido)pentyl)Ureido)-pentanedioic acid for PSMA-targeted α-particle radiopharmaceutical therapy. J Nucl Med. 2016;57:1569–75.
Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer AUA/ASTRO/SUO Guideline. Part I: Risk stratification, shared decision making, and care options. J Urol. 2018;199:683–90.
Trabulsi EJ, Rumble RB, Jadvar H, et al. Optimum imaging strategies for advanced prostate cancer: ASCO guideline. J Clin Oncol. 2020;38:1963–96.
Hövels AM, Heesakkers RAM, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63:387–95.
Bostwick DG, Pacelli A, Blute M, et al. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998;82:2256–61.
Chang SS. Overview of prostate-specific membrane antigen. Rev Urol. 2004;6(10):S13–8.
Afshar-Oromieh A, Haberkorn U, Eder M, et al. [68Ga] Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18 F-FECH. Eur J Nucl Med Mol Imaging. 2012;39:1085–6.
Afshar-Oromieh A, Malcher A, Eder M, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–95.
Fendler WP, Calais J, Allen-Auerbach M, et al. 68Ga-PSMA-11 PET/CT Interobserver agreement or prostate cancer assessments: an international multicenter prospective study. J Nucl Med. 2017 ;58(10):1617–23.
Mattiolli AB, Santos A, Vicente A, et al. Impact of 68GA-PSMA PET/CT on treatment of patients with recurrent/metastatic high risk prostate cancer—a multicenter study. Int Braz J Urol. 2018;44(5):892–9.
Morigi JJ, Stricker PD, van Leeuwen PJ, et al. Prospective comparison of 18Ffluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–90.
Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5:856–63.
Kabasakal L, Demirci E, Nematyazar J, et al. The role of PSMA PET/CT imaging in restaging of prostate cancer patients with low prostate-specific antigen levels. Nucl Med Commun. 2017;38:149–55.
Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet (Lon, Engl). 2020;395:1208–16.
Carlucci G, Ippisch R, Slavik R, et al. 68Ga-PSMA-11 NDA approval: a novel and successful academicpartnership. J Nucl Med. 2021;62:149–55. https://doi.org/10.2967/jnumed.120.260455.
Chen Y, Pullambhatla M, Foss CA, Byun Y, Nimmagadda S, Senthamizhchelvan S, Sgouros G, Mease RC, Pomper MG. 2-(3-{1-Carboxy-5-[(6-[18F] fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid,[18F] DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res. 2011;17(24):7645–53.
Liu W, Zukotynski K, Emmett L, et al. A prospective study of 18F-DCFPyL PSMA PET/CT restaging in recurrent prostate cancer following primary external beam radiotherapy or brachytherapy. Int J Radiat Oncol Biol Phys. 2020;106:546–55.
Giesel FL, Will L, Lawal I, et al. Intraindividual Comparison of (18)F-PSMA-1007 and (18)FDCFPyL PET/CT in the Prospective evaluation of patients with newly diagnosed prostate carcinoma: a pilot study. J Nucl Med. 2018;59:1076–80.
Giesel FL, Will L, Lawal I, et al. Intraindividual comparison of 18F-PSMA-1007 and 18FDCFPyL PET/CT in the prospective evaluation of patients with Newly diagnosed prostate carcinoma: a pilot study. J Nucl Med. 2018;59(7):1076–80.
Scarpa L, Buxbaum S, Kendler D, et al. The (68)Ga/(177)Lu theragnostic concept in PSMA targeting of castration-resistant prostate cancer: correlation of SUVmax values and absorbed dose estimates. Eur J Nucl Med Mol Imaging. 2017;44:788–800.
Ahmadzadehfar H, Rahbar K, Kurpig S, et al. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res. 2015;5:114.
Yadav MP, Ballal S, Tripathi M, et al. (177)Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: Safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol Imaging. 2017;44:81–91.
von Eyben FE, Roviello G, Kiljunen T, et al. Third-line treatment and 177Lu-PSMA radioligand therapy of metastatic castration-resistant prostate cancer: a systematic review. Eur J Nucl Med Mol Imaging. 2018;45(3):496–508.
Calopedos RJS, Chalasani V, Asher R, et al. Lutetium-177-labelled anti-prostate-specific membrane antigen antibody and ligands for the treatment of metastatic castrate-resistant prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017;20:352–60.
Hofman MS, Emmett L, Violet J, et al. TheraP: a randomized phase 2 trial of (177)Lu-PSMA-617 theranostic treatment vs cabazitaxel in progressive metastatic castration-resistant prostate cancer (Clinical Trial Protocol ANZUP 1603). BJU Int. 2019;124(1):5–13.
Hofman M, Violet J, Hicks R, et al. Results of a 50 patient single-center phase II prospective trial of Lutetium-177 PSMA-617 theranostics in metastatic castrate-resistant prostate cancer. J Clin Oncol. 2019;37(7):228–228.
Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018 ;19(6):825–33.
Calais J, Fendler W, Eiber M, et al. RESIST-PC phase 2 trial: 177Lu-PSMA-617 radionuclide therapy for metastatic castrate-resistant prostate cancer. J Clin Oncol. 2019;37(15):5028–5028.
Privé BM, Peters SMB, Muselaers CHJ, et al. Lutetium-177-PSMA-617 in low-volume hormone sensitive metastatic prostate cancer, a prospective pilot study. Clin Cancer Res. 2021. https://doi.org/10.1158/1078-0432.CCR-20-4298.
Privé BM, et al. Lutetium-177-PSMA-I&T as metastases directed therapy in oligometastatic hormone sensitive prostate cancer, a randomized controlled trial. BMC Cancer. 2020;201:884.
Emmett L, Subramaniam S, Zhang AY, et al. ENZA-p: a randomized phase II trial using PSMA as a therapeutic agent and prognostic indicator in men with metastatic castration-resistant prostate cancer treated with enzalutamide (ANZUP 1901). J Clin OncoL. 2021;39(6):177–177.
Ahmadzadehfar H. Targeted therapy for metastatic prostate cancer with radionuclides. Prostate cancer—leading-edge diagnostic procedures and treatments. 2016.https://www.intechopen.com/books/prostate-cancer-leading-edge-diagnostic-procedures-andtreatments/targeted-therapy-for-metastatic-prostate-cancer-with-radionuclides. Accessed 10 Feb 2021.
Gröner D, Ngoc CN, Davis K, et al. Radioligand therapy with 177Lu-PSMA-617 in patients with diffuse bone marrow involvement: safety and efficacy results. J Nucl Med. 2020;61(1):1280.
Sgouros G, Bodei L, McDevitt MR, et al. Radiopharmaceutical therapy in cancer: clinical advances and challenges. Nat Rev Drug Discov. 2020;19:589–608.
Larson SM, Carrasquillo JA, Cheung NK, Press OW. Radioimmunotherapy of human tumours. Nat Rev Cancer. 2015;15:347–60.
Kratochwil C, Bruchertseifer F, Giesel FL, et al. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med. 2016;57:1941–4.
Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted alpha therapy of mCRPC with 225actinium-PSMA-617: dosimetry estimate and empirical dose finding. J Nucl Med. 2017;58:1624–31.
Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with (225)Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med. 2018;59:795–802.
Yadav M, Ballal S, Bal C, et al. Clinical experience on 225Ac-PSMA-617 targeted α-therapy in metastatic castration resistant prostate cancer patients: safety and efficacy results. J Nucl Med. 2020;61(1):589.
van der Doelen MJ, Mehra N, Smits M, et al. Clinical experience with PSMA-Actinium-225 (Ac-225) radioligand therapy (RLT) in end-stage metastatic castration-resistant prostate cancer (mCRPC) patients. J Clin Oncol. 2018;36(6):344–344.
Kratochwil C, Bruchertseifer F, Giesel FL, et al. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med. 2016 ;57(12):1941–4.
Kiess AP, Minn I, Vaidyanathan G, et al. (2S)-2-(3-(1-carboxy-5-(4–211Atastatobenzamido) pentyl)ureido)-pentanedioic acid for PSMA-targeted alpha-particle radiopharmaceutical therapy. J Nucl Med. 2016;57:1569–75.
Hammer S, Larssen A, Ellingsen C, et al. Preclinical pharmacology of the PSMA-targeted thorium-227 conjugate PSMA-TTC: a novel targeted alpha therapeutic for the treatment of prostate cancer. Cancer Res. 2017. https://doi.org/10.1158/1538-7445.AM2017-5200.
Hammer S., Hagemann U.B., Zitzmann-Kolbe S., et al. Preclinical activity of PSMA-TTC, a targeted alpha therapeutic in patient-derived prostate cancer models. Cancer Res. 2018; 78
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The following authors have no disclosures: Meghana Parsi, Milap H. Desai, Devashish Desai, Sachi Singhal, Pushti Khandwala, Rashmika R. Potdar.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Parsi, M., Desai, M.H., Desai, D. et al. PSMA: a game changer in the diagnosis and treatment of advanced prostate cancer. Med Oncol 38, 89 (2021). https://doi.org/10.1007/s12032-021-01537-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-021-01537-3