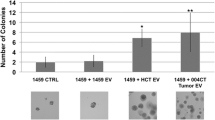
Abstract
Linoleic acid (LA) is the most abundant polyunsaturated fatty acid in occidental diets, which mediate a variety of processes in human breast cancer cells, including migration and invasion. Extracellular vesicles (EVs) are vesicles released from endosomes and plasma membrane that are composed of a variety of molecules, including proteins, nucleic acids and lipids. EVs from cancer cells promote processes related with cancer progression. In the present study, we demonstrate that treatment of MDA-MB-231 cells with EVs from MDA-MB-231 cells stimulated with LA (LA EVs) promote migration and invasion via Src activity. LA EVs induce activation of FAK via Src activity and of Src and Akt2. LA EVs also induce the assembly of focal adhesions and MMP-9 secretion. These findings demonstrate that LA EVs mediate an autocrine and/or paracrine Src/FAK signaling pathway to promote migration and invasion.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36(9):1007–24. https://doi.org/10.1007/s11745-001-0812-7.
Diaz-Aragon R, Ramirez-Ricardo J, Cortes-Reynosa P, Simoni-Nieves A, Gomez-Quiroz LE, Perez SE. Role of phospholipase D in migration and invasion induced by linoleic acid in breast cancer cells. Mol Cell Biochem. 2019;457(1–2):119–32. https://doi.org/10.1007/s11010-019-03517-8.
Espinosa-Neira R, Mejia-Rangel J, Cortes-Reynosa P, Salazar EP. Linoleic acid induces an EMT-like process in mammary epithelial cells MCF10A. Int J Biochem Cell Biol. 2011;43(12):1782–91. https://doi.org/10.1016/j.biocel.2011.08.017.
Serna-Marquez N, Diaz-Aragon R, Reyes-Uribe E, Cortes-Reynosa P, Salazar EP. Linoleic acid induces migration and invasion through FFAR4- and PI3K-/Akt-dependent pathway in MDA-MB-231 breast cancer cells. Med Oncol. 2017;34(6):111. https://doi.org/10.1007/s12032-017-0969-3.
Serna-Marquez N, Villegas-Comonfort S, Galindo-Hernandez O, Navarro-Tito N, Millan A, Salazar EP. Role of LOXs and COX-2 on FAK activation and cell migration induced by linoleic acid in MDA-MB-231 breast cancer cells. Cell Oncol (Dordr). 2013;36(1):65–77. https://doi.org/10.1007/s13402-012-0114-4.
Lee TH, Chennakrishnaiah S, Audemard E, Montermini L, Meehan B, Rak J. Oncogenic ras-driven cancer cell vesiculation leads to emission of double-stranded DNA capable of interacting with target cells. Biochem Biophys Res Commun. 2014;451(2):295–301. https://doi.org/10.1016/j.bbrc.2014.07.109.
Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10(5):619–24. https://doi.org/10.1038/ncb1725.
Fedele C, Singh A, Zerlanko BJ, Iozzo RV, Languino LR. The alphavbeta6 integrin is transferred intercellularly via exosomes. J Biol Chem. 2015;290(8):4545–51. https://doi.org/10.1074/jbc.C114.617662.
Brzozowski JS, Jankowski H, Bond DR, McCague SB, Munro BR, Predebon MJ, et al. Lipidomic profiling of extracellular vesicles derived from prostate and prostate cancer cell lines. Lipids Health Dis. 2018;17(1):211. https://doi.org/10.1186/s12944-018-0854-x.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83. https://doi.org/10.1083/jcb.201211138.
Abak A, Abhari A, Rahimzadeh S. Exosomes in cancer: small vesicular transporters for cancer progression and metastasis, biomarkers in cancer therapeutics. PeerJ. 2018;6:e4763. https://doi.org/10.7717/peerj.4763.
Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W. Exosomes in cancer: small particle, big player. J Hematol Oncol. 2015;8:83. https://doi.org/10.1186/s13045-015-0181-x.
Wang HX, Gires O. Tumor-derived extracellular vesicles in breast cancer: from bench to bedside. Cancer Lett. 2019;460:54–64. https://doi.org/10.1016/j.canlet.2019.06.012.
Galindo-Hernandez O, Serna-Marquez N, Castillo-Sanchez R, Salazar EP. Extracellular vesicles from MDA-MB-231 breast cancer cells stimulated with linoleic acid promote an EMT-like process in MCF10A cells. Prostaglandins Leukot Essent Fatty Acids. 2014;91(6):299–310. https://doi.org/10.1016/j.plefa.2014.09.002.
Leal-Orta E, Ramirez-Ricardo J, Cortes-Reynosa P, Galindo-Hernandez O, Salazar EP. Role of PI3K/Akt on migration and invasion of MCF10A cells treated with extracellular vesicles from MDA-MB-231 cells stimulated with linoleic acid. J Cell Commun Signal. 2019. https://doi.org/10.1007/s12079-018-0490-2.
Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6(2):154–61. https://doi.org/10.1038/ncb1094ncb1094.
Bellis SL, Miller JT, Turner CE. Characterization of tyrosine phosphorylation of paxillin in vitro by focal adhesion kinase. J Biol Chem. 1995;270(29):17437–41.
Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18(5):516–23. https://doi.org/10.1016/j.ceb.2006.08.011.
Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15(2):954–63.
Ramirez-Ricardo J, Leal-Orta E, Martinez-Baeza E, Ortiz-Mendoza C, Breton-Mora F, Herrera-Torres A, et al. Circulating extracellular vesicles from patients with breast cancer enhance migration and invasion via a Srcdependent pathway in MDAMB231 breast cancer cells. Mol Med Rep. 2020;22(3):1932–48. https://doi.org/10.3892/mmr.2020.11259.
Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271(2):695–701.
Chajes V, Torres-Mejia G, Biessy C, Ortega-Olvera C, Angeles-Llerenas A, Ferrari P, et al. Omega-3 and omega-6 Polyunsaturated fatty acid intakes and the risk of breast cancer in Mexican women: impact of obesity status. Cancer Epidemiol Biomark Prev. 2012;21(2):319–26. https://doi.org/10.1158/1055-9965.EPI11-0896.
Murff HJ, Shu XO, Li H, Yang G, Wu X, Cai H, et al. Dietary polyunsaturated fatty acids and breast cancer risk in Chinese women: a prospective cohort study. Int J Cancer. 2011;128(6):1434–41. https://doi.org/10.1002/ijc.25703.
Azrad M, Turgeon C, Demark-Wahnefried W. Current evidence linking polyunsaturated fatty acids with cancer risk and progression. Front Oncol. 2013;3:224. https://doi.org/10.3389/fonc.2013.00224.
Khadge S, Thiele GM, Sharp JG, McGuire TR, Klassen LW, Black PN, et al. Long-chain omega-3 polyunsaturated fatty acids decrease mammary tumor growth, multiorgan metastasis and enhance survival. Clin Exp Metastasis. 2018;35(8):797–818. https://doi.org/10.1007/s10585-018-9941-7.
Hannafon BN, Carpenter KJ, Berry WL, Janknecht R, Dooley WC, Ding WQ. Exosome-mediated microRNA signaling from breast cancer cells is altered by the anti-angiogenesis agent docosahexaenoic acid (DHA). Mol Cancer. 2015;14:133. https://doi.org/10.1186/s12943-015-0400-7.
Villegas-Comonfort S, Castillo-Sanchez R, Serna-Marquez N, Cortes-Reynosa P, Salazar EP. Arachidonic acid promotes migration and invasion through a PI3K/Akt-dependent pathway in MDA-MB-231 breast cancer cells. Prostaglandins Leukot Essent Fatty Acids. 2014;90(5):169–77. https://doi.org/10.1016/j.plefa.2014.01.007.
Reyes N, Reyes I, Tiwari R, Geliebter J. Effect of linoleic acid on proliferation and gene expression in the breast cancer cell line T47D. Cancer Lett. 2004;209(1):25–35. https://doi.org/10.1016/j.canlet.2003.12.010.
Senatorov IS, Moniri NH. The role of free-fatty acid receptor-4 (FFA4) in human cancers and cancer cell lines. Biochem Pharmacol. 2018;150:170–80. https://doi.org/10.1016/j.bcp.2018.02.011.
Yonezawa T, Katoh K, Obara Y. Existence of GPR40 functioning in a human breast cancer cell line, MCF-7. Biochem Biophys Res Commun. 2004;314(3):805–9. https://doi.org/10.1016/j.bbrc.2003.12.175.
Jiang P, Enomoto A, Takahashi M. Cell biology of the movement of breast cancer cells: intracellular signalling and the actin cytoskeleton. Cancer Lett. 2009;284(2):122–30. https://doi.org/10.1016/j.canlet.2009.02.034.
Polyak K, Kalluri R. The role of the microenvironment in mammary gland development and cancer. Cold Spring Harb Perspect Biol. 2010;2(11):a003244. https://doi.org/10.1101/cshperspect.a003244.
Dolo V, Ginestra A, Cassara D, Violini S, Lucania G, Torrisi MR, et al. Selective localization of matrix metalloproteinase 9, beta1 integrins, and human lymphocyte antigen class I molecules on membrane vesicles shed by 8701-BC breast carcinoma cells. Cancer Res. 1998;58(19):4468–74.
Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123(Pt 10):1603–11. https://doi.org/10.1242/jcs.064386.
DeRita RM, Zerlanko B, Singh A, Lu H, Iozzo RV, Benovic JL, et al. c-Src, insulin-like growth factor I receptor, G-protein-coupled receptor kinases and focal adhesion kinase are enriched into prostate cancer cell exosomes. J Cell Biochem. 2017;118(1):66–73. https://doi.org/10.1002/jcb.25611.
Galindo-Hernandez O, Villegas-Comonfort S, Candanedo F, Gonzalez-Vazquez MC, Chavez-Ocana S, Jimenez-Villanueva X, et al. Elevated concentration of microvesicles isolated from peripheral blood in breast cancer patients. Arch Med Res. 2013;44(3):208–14. https://doi.org/10.1016/j.arcmed.2013.03.002.
Fuentes P, Sese M, Guijarro PJ, Emperador M, Sanchez-Redondo S, Peinado H, et al. ITGB3-mediated uptake of small extracellular vesicles facilitates intercellular communication in breast cancer cells. Nat Commun. 2020;11(1):4261. https://doi.org/10.1038/s41467-020-18081-9.
Acknowledgements
We are grateful to Nora Ruiz and María de Lourdes-Rojas (LaNSE, Cinvestav-IPN) for their technical assistance.
Funding
This research was funded by CONACYT (255429) and CONACYT-FOSISS (Salud 2015-1-261637), Mexico. Grants from CONACYT supported JRR, ELO, AGH and RDA.
Author information
Authors and Affiliations
Contributions
JR-R designed the study and wrote the manuscript. JR-R and EL-O performed the experiments and analyses. AG-H, RD-A and PC-R validated the data and reviewed/edited the manuscript. EPS, RT-B and JR-R coordinated the study and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12032_2021_1485_MOESM1_ESM.tif
Fig. S1. Schematic diagram showing the experimental procedure of stimulation of MDA-MB-231 with 90 μM LA for different periods of time (12, 24, 36 and 48 h). (TIF 1120 kb)
12032_2021_1485_MOESM2_ESM.tif
Fig. S2. Schematic diagram showing the experimental procedure of treatment for 6, 9, 12, 18 and/or 24 h of MDA-MB-231 cells with Ctrl EVs and LA EVs. (TIF 1436 kb)
Rights and permissions
About this article
Cite this article
Ramirez-Ricardo, J., Leal-Orta, E., Garcia-Hernandez, A. et al. Role of Src/FAK in migration and invasion mediated by extracellular vesicles from MDA-MB-231 cells stimulated with linoleic acid. Med Oncol 38, 40 (2021). https://doi.org/10.1007/s12032-021-01485-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-021-01485-y