Abstract
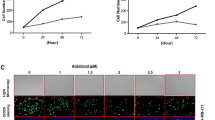
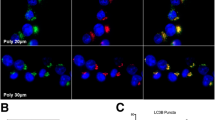
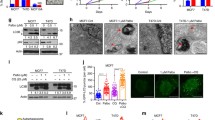
Non-visual arrestins (β-arrestins) are endocytic proteins that mediate agonist-activated GPCRs internalization and signaling pathways in an independent manner. The involvement of β-arrestins in cancer invasion and metastasis is increasingly reported. So, it is hypothesized that inhibition of β-arrestins may diminish the survival chances of cancer cells. This study aimed to evaluate the in vitro impact of inhibiting β-arrestins on the autophagic and/or apoptotic responsiveness of breast cancer cells. We used Barbadin to selectively inhibit β-Arr/AP2 interaction in AVP-stimulated V2R receptor of triple-negative breast cancer cells (MDA MB-231). Autophagy was assessed by the microtubule-associated protein 1 light chain 3-II (LC3II), apoptosis was measured by Annexin-V/PI staining and cell cycle distribution was investigated based upon the DNA content using flow cytometry. Barbadin reduced cell viability to 69.1% and increased the autophagy marker LC3II and its autophagic effect disappeared in cells transiently starved in Earle's balanced salt solution (EBSS). Also, Barbadin mildly enhanced the expression of P62 mRNA and arrested 63.7% of cells in G0/G1 phase. In parallel, the drug-induced apoptosis in 29.9% of cells (by AV/PI) and 27.8% of cells were trapped in sub-G1 phase. The apoptotic effect of Barbadin was enhanced when autophagy was inhibited by the PI3K inhibitor (Wortmannin). Conclusively, the data demonstrate the dual autophagic and apoptotic effects of β-βArr/AP2 inhibition in triple-negative breast cancer cells. These observations nominate β-Arrs as selective targets in breast cancer treatment.






Similar content being viewed by others
References
Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ. 2005;12(2):1528–34.
Gewirtz DA. The four faces of autophagy: implications for cancer therapy. Can Res. 2014;74(3):647–51.
Eisenberg-Lerner A, Bialik S, Simon H-U, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16(7):966–75.
Gurevich VV, Gurevich EV. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol Ther. 2006;110(3):465–502.
Kang Y, Zhou XE, Gao X, He Y, Liu W, Ishchenko A, Barty A, White TA, Yefanov O, Han GW. Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature. 2015;523(7562):561–7.
Goodman OB, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. β-Arrestin acts as a clathrin adaptor in endocytosis of the β 2-adrenergic receptor. Nature. 1996;383(6599):447–50.
Luttrell L, Ferguson S, Daaka Y, Miller W, Maudsley S, Della Rocca G, Lin F-T, Kawakatsu H, Owada K, Luttrell D. β-Arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283(5402):655–61.
Jean-Charles PY, Kaur S, Shenoy SK. GPCR signaling via β-arrestin-dependent mechanisms. J Cardiovasc Pharmacol. 2017;70(3):142.
Song Q, Ji Q, Li Q. The role and mechanism of β-arrestins in cancer invasion and metastasis. Int J Mol Med. 2018;41(2):631–9.
Shukla AK, Dwivedi-Agnihotri H. Structure and function of β-arrestins, their emerging role in breast cancer, and potential opportunities for therapeutic manipulation. Adv Cancer Res Elsevier. 2020;145:139–56.
Wahba HA, El-Hadaad HA. Current approaches in treatment of triple-negative breast cancer. Cancer Biol Med. 2015;12(2):106.
Ge L, Shenoy SK, Lefkowitz RJ, DeFea K. Constitutive protease-activated receptor-2-mediated migration of MDA MB-231 breast cancer cells requires both β-arrestin-1 and-2. J Biol Chem. 2004;279(53):55419–24.
Feigin ME, Xue B, Hammell MC, Muthuswamy SK. G-protein–coupled receptor GPR161 is overexpressed in breast cancer and is a promoter of cell proliferation and invasion. Proc Natl Acad Sci. 2014;111(11):4191–6.
Jing X, Zhang H, Hu J, Su P, Zhang W, Jia M, Cheng H, Li W, Zhou G. β-arrestin 2 is associated with multidrug resistance in breast cancer cells through regulating MDR1 gene expression. Int J Clin Exp Pathol. 2015;8(2):1354.
Cailleau R, Young R, Olive M, Reeves W Jr. Breast tumor cell lines from pleural effusions. J Natl Cancer Inst. 1974;53(3):661–74.
Beautrait A, Paradis JS, Zimmerman B, Giubilaro J, Nikolajev L, Armando S, Kobayashi H, Yamani L, Namkung Y, Heydenreich FM. A new inhibitor of the β-arrestin/AP2 endocytic complex reveals interplay between GPCR internalization and signalling. Nat Commun. 2017;8(1):1–16.
Charest PG, Oligny-Longpré G, Bonin H, Azzi M, Bouvier M. The V2 vasopressin receptor stimulates ERK1/2 activity independently of heterotrimeric G protein signalling. Cell Signal. 2007;19(1):32–41.
Liu WJ, Ye L, Huang WF, Guo LJ, Xu ZG, Wu HL, Yang C, Liu HF. p62 links the autophagy pathway and the ubiqutin–proteasome system upon ubiquitinated protein degradation. Cell Mol Biol Lett. 2016;21(1):29.
Garona J, Pifano M, Orlando UD, Pastrian MB, Iannucci NB, Ortega HH, Podesta EJ, Gomez DE, Ripoll GV, Alonso DF. The novel desmopressin analogue [V4Q5] dDAVP inhibits angiogenesis, tumour growth and metastases in vasopressin type 2 receptor-expressing breast cancer models. Int J Oncol. 2015;46(6):2335–45.
Cui J, Lu K, Shi Y, Chen B, Tan S-H, Gong Z, Shen H-M. Integrated and comparative miRNA analysis of starvation-induced autophagy in mouse embryonic fibroblasts. Gene. 2015;571(2):194–204.
Chiu C-F, Chin H-K, Huang W-J, Bai L-Y, Huang H-Y, Weng J-R. Induction of Apoptosis and Autophagy in Breast Cancer Cells by a Novel HDAC8 Inhibitor. Biomolecules. 2019;9(12):824.
Zhu W, Qu H, Xu K, Jia B, Li H, Du Y, Liu G, Wei HJ, Zhao HY. Differences in the starvation-induced autophagy response in MDA-MB-231 and MCF-7 breast cancer cells. Anim Cells Syst (Seoul). 2017;21(3):190–8.
Ichimura Y, Komatsu M. Selective degradation of p62 by autophagy. Paper Present Sem Immunopathol. 2010;32(4):431–6.
Komatsu M, Ichimura Y. Physiological significance of selective degradation of p62 by autophagy. FEBS Lett. 2010;584(7):1374–8.
Thompson HGR, Harris JW, Wold BJ, Lin F, Brody JP. p62 overexpression in breast tumors and regulation by prostate-derived Ets factor in breast cancer cells. Oncogene. 2003;22(15):2322–33.
Li J, Li F, Wang H, Wang X, Jiang Y, Li D. Wortmannin reduces metastasis and angiogenesis of human breast cancer cells via nuclear factor-κB-dependent matrix metalloproteinase-9 and interleukin-8 pathways. J Int Med Res. 2012;40(3):867–76.
Wu Y-T, Tan H-L, Shui G, Bauvy C, Huang Q, Wenk MR, Ong C-N, Codogno P, Shen H-M. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem. 2010;285(14):10850–61.
Akter R, Hossain MZ, Kleve MG, Gealt MA. Wortmannin induces MCF-7 breast cancer cell death via the apoptotic pathway, involving chromatin condensation, generation of reactive oxygen species, and membrane blebbing. Breast Cancer Targets Therapy. 2012;4:103.
Ahn S, Kim J, Hara MR, Ren X-R, Lefkowitz RJ. β-Arrestin-2 mediates anti-apoptotic signaling through regulation of BAD phosphorylation. J Biol Chem. 2009;284(13):8855–65.
Kook S, Gurevich VV, Gurevich EV. Arrestins in apoptosis. Arrestins-Pharmacology and Therapeutic Potential: Springer; 2014. p. 309–39.
Wang P, Xu T-Y, Wei K, Guan Y-F, Wang X, Xu H, Su D-F, Pei G, Miao C-Y. ARRB1/β-arrestin-1 mediates neuroprotection through coordination of BECN1-dependent autophagy in cerebral ischemia. Autophagy. 2014;10(9):1535–48.
Seo J, Tsakem EL, Breitman M, Gurevich VV. Identification of arrestin-3-specific residues necessary for JNK3 kinase activation. J Biol Chem. 2011;286(32):27894–901.
Shin WH, Park JH, Chung KC. The central regulator p62 between ubiquitin proteasome system and autophagy and its role in the mitophagy and Parkinson’s disease. BMB Rep. 2020;53(1):56.
Liu Y-L, Chou C-K, Kim M, Vasisht R, Kuo Y-A, Ang P, Liu C, Perillo EP, Chen Y-A, Blocher K. Assessing metastatic potential of breast cancer cells based on EGFR dynamics. Sci Rep. 2019;9(1):1–13.
Lundgren K, Tobin NP, Lehn S, Stål O, Rydén L, Jirström K, Landberg G. Stromal expression of β-arrestin-1 predicts clinical outcome and tamoxifen response in breast cancer. J Mol Diagn. 2011;13(3):340–51.
Park SY, Jun J, Jeong KJ, Heo HJ, Sohn JS, Lee HY, Park CG, Kang J. Histone deacetylases 1, 6 and 8 are critical for invasion in breast cancer. Oncol Rep. 2011;25(6):1677–81.
Bai Y, Chen Y, Chen X, Jiang J, Wang X, Wang L, Wang J, Zhang J, Gao L. Trichostatin A activates FOXO1 and induces autophagy in osteosarcoma. Arch Med Sci AMS. 2019;15(1):204.
Alao JP, Lam EW, Ali S, Buluwela L, Bordogna W, Lockey P, Varshochi R, Stavropoulou AV, Coombes RC, Vigushin DM. Histone deacetylase inhibitor trichostatin A represses estrogen receptor α-dependent transcription and promotes proteasomal degradation of cyclin D1 in human breast carcinoma cell lines. Clin Cancer Res. 2004;10(23):8094–104.
Acknowledgement
Authors would like to express their gratitude toward Science and Technology Development Fund (STDF), for providing the financial supported to this work (grant number 34850).
Author information
Authors and Affiliations
Contributions
Conceptualization: MH and TD; Methodology and investigations: MA, MK, TD and MH; Editing the original draft and revision: MH, MK and TD; Project administration, MH; Funding acquisition, MH, TD, MK.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Donia, T., Abouda, M., Kelany, M. et al. β-Arrestin inhibition induces autophagy, apoptosis, G0/G1 cell cycle arrest in agonist-activated V2R receptor in breast cancer cells. Med Oncol 38, 38 (2021). https://doi.org/10.1007/s12032-021-01484-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-021-01484-z