Abstract
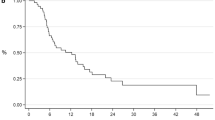
We conducted a risk-adapted upfront docetaxel (DOC) in patients with metastatic hormone-sensitive prostate cancer (mHSPC). Here, we reported an interim analysis of the study. The study enrolled 68 patients with newly diagnosed mHSPC between 2016 and 2018. According to the presence of visceral metastasis, an EOD score ≥ 3, or prostate-specific antigen (PSA) level at 3 months of ≥ 1 ng/mL, patients were divided into low- and high-risk groups. Patients were treated with androgen deprivation therapy (ADT) with or without bicalutamide; those in the high-risk group received upfront treatment involving six cycles of DOC (70 mg/m2). Short-term treatment effect, adverse events, and quality of life (QOL) were evaluated. Fifty (73.5%) were classified in the high-risk group, and 46 (67%) received upfront ADT + DOC. In the ADT + DOC group, 43.5% (20/46) patients achieved a PSA level ≤ 0.2 ng/mL. PSA nadir and time to PSA nadir were 0.291 ng/mL and 288 days, respectively. In the ADT + DOC group, 76.1% (35/42) patients had adverse events (AEs) of grade ≥ 3. During a median follow-up of 18.5 months, 36.4% (8/22) patients in the ADT group and 43.5% (20/46) in the ADT + DOC group had CRPC. Two QOL scores including the physical status and appetite loss at 6 months significantly worsened in the ADT + DOC group but was resolved by 12 months. Upfront DOC achieved high PSA responses without long-term QOL deterioration. However, the short-term outcomes were limited. Longer follow-up is needed to determine the survival advantage.



Similar content being viewed by others
References
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–53. https://doi.org/10.1002/ijc.31937.
Tsao CK, Oh WK. First-line treatment of hormone-sensitive metastatic prostate cancer: is there a single standard of care? J Clin Oncol. 2018. https://doi.org/10.1200/jco.2017.77.4315.
Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, Dreicer R, Vogelzang NJ, Picus J, Shevrin D, Hussain M, Garcia JA, DiPaola RS. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–46. https://doi.org/10.1056/NEJMoa1503747.
Barata PC, Sartor AO. Metastatic castration-sensitive prostate cancer: abiraterone, docetaxel, or. Cancer. 2019;125(11):1777–88. https://doi.org/10.1002/cncr.32039.
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Ozguroglu M, Ye D, Feyerabend S, Protheroe A, De Porre P, Kheoh T, Park YC, Todd MB, Chi KN. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352–60. https://doi.org/10.1056/NEJMoa1704174.
Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, Ritchie AWS, Attard G, Chowdhury S, Cross W, Dearnaley DP, Gillessen S, Gilson C, Jones RJ, Langley RE, Malik ZI, Mason MD, Matheson D, Millman R, Russell JM, Thalmann GN, Amos CL, Alonzi R, Bahl A, Birtle A, Din O, Douis H, Eswar C, Gale J, Gannon MR, Jonnada S, Khaksar S, Lester JF, O’Sullivan JM, Parikh OA, Pedley ID, Pudney DM, Sheehan DJ, Srihari NN, Tran ATH, Parmar MKB, Sydes MR. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018. https://doi.org/10.1016/s0140-6736(18)32486-3.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, Coskinas X, Frydenberg M, Hague WE, Horvath LG, Joshua AM, Lawrence NJ, Marx G, McCaffrey J, McDermott R, McJannett M, North SA, Parnis F, Parulekar W, Pook DW, Reaume MN, Sandhu SK, Tan A, Tan TH, Thomson A, Tu E, Vera-Badillo F, Williams SG, Yip S, Zhang AY, Zielinski RR, Sweeney CJ, Investigators ET, the A, New Zealand U, Prostate Cancer Trials G. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121–31. https://doi.org/10.1056/NEJMoa1903835.
Narita S, Hatakeyama S, Takahashi M, Sakurai T, Kawamura S, Hoshi S, Ishida M, Kawaguchi T, Ishidoya S, Shimoda J, Sato H, Koizumi A, Mitsuzuka K, Tochigi T, Tsuchiya N, Ohyama C, Arai Y, Nomura K, Habuchi T. Clinical outcomes and prognostic factors in patients with newly diagnosed metastatic prostate cancer initially treated with androgen deprivation therapy: a retrospective multicenter study in Japan. Int J Clin Oncol. 2020. https://doi.org/10.1007/s10147-019-01614-8.
Akamatsu S, Kubota M, Uozumi R, Narita S, Takahashi M, Mitsuzuka K, Hatakeyama S, Sakurai T, Kawamura S, Ishidoya S, Hoshi S, Ishida M, Mizuno K, Ogura K, Goto T, Terada N, Kobayashi T, Yamasaki T, Inoue T, Tsuchiya N, Ohyama C, Arai Y, Habuchi T, Morita S, Ogawa O. Development and validation of a novel prognostic model for predicting overall survival in treatment-naïve castration-sensitive metastatic prostate cancer. Eur Urol Oncol. 2019;2(3):320–8. https://doi.org/10.1016/j.euo.2018.10.011.
Kwon WA, Joung JY, Lee JE, Choi SY, Kim SH, Seo HK, Lee KH, Kim CS. Use of docetaxel plus androgen deprivation therapy for metastatic hormone-sensitive prostate cancer in Korean patients: a retrospective study. Investig Clin Urol. 2019;60(3):195–201. https://doi.org/10.4111/icu.2019.60.3.195.
Soloway MS, Hardeman SW, Hickey D, Raymond J, Todd B, Soloway S, Moinuddin M. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1988;61(1):195–202.
Sato H, Narita S, Tsuchiya N, Koizumi A, Nara T, Kanda S, Numakura K, Tsuruta H, Maeno A, Saito M, Inoue T, Satoh S, Nomura K, Habuchi T. Impact of early changes in serum biomarkers following androgen deprivation therapy on clinical outcomes in metastatic hormone-sensitive prostate cancer. BMC Urol. 2018;18(1):32. https://doi.org/10.1186/s12894-018-0353-4.
Flaig TW, Plets M, Hussain MHA, Agarwal N, Mitsiades N, Deshpande HA, Vaishampayan UN, Thompson IM Jr. Abiraterone acetate for metastatic prostate cancer in patients with suboptimal biochemical response to hormone induction. JAMA Oncol. 2017;3(11):e170231. https://doi.org/10.1001/jamaoncol.2017.0231.
Graff JN, Stein MN, Surana R, Al Rabadi L, Liu E, Fong L, Bailey S, Latour E, Newby TA, Moran AE, Beer TM. Phase II study of ipilimumab in men with metastatic prostate cancer with an incomplete response to androgen deprivation therapy. Front Oncol. 2020;10:1381. https://doi.org/10.3389/fonc.2020.01381.
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148–59.
Harshman LC, Chen YH, Liu G, Carducci MA, Jarrard D, Dreicer R, Hahn N, Garcia JA, Hussain M, Shevrin D, Eisenberger M, Kohli M, Plimack ER, Cooney M, Vogelzang NJ, Picus J, Dipaola R, Sweeney CJ, Investigators E-A. Seven-month prostate-specific antigen is prognostic in metastatic hormone-sensitive prostate cancer treated with androgen deprivation with or without docetaxel. J Clin Oncol. 2018;36(4):376–82. https://doi.org/10.1200/jco.2017.75.3921.
Rulach RJ, McKay S, Neilson S, White L, Wallace J, Carruthers R, Lamb C, Cascales A, Marashi H, Glen H, Venugopal B, Sadoyze A, Sidek N, Russell JM, Alhasso A, Dodds D, Laskey J, Jones RJ, MacLeod N. Real-world uptake, safety profile and outcomes of docetaxel in newly diagnosed metastatic prostate cancer. BJU Int. 2018;121(2):268–74. https://doi.org/10.1111/bju.14025.
Barata P, Emamekhoo H, Mendiratta P, Koshkin V, Tyler A, Ornstein M, Rini BI, Gilligan T, Kyriakopoulos C, Garcia JA. Treatment selection for men with metastatic prostate cancer who progress on upfront chemo-hormonal therapy. Prostate. 2018;78(13):1035–41. https://doi.org/10.1002/pros.23663.
Mager R, Savko O, Bohm K, Thomas A, Dotzauer R, Borgmann H, Jager W, Thomas C, Haferkamp A, Hofner T, Tsaur I. Comparative assessment of docetaxel for safety and efficacy between hormone-sensitive and castration-resistant metastatic prostate cancer. Urol Oncol. 2019;37(12):999–1005. https://doi.org/10.1016/j.urolonc.2019.07.005.
Shiota M, Yokomizo A, Takeuchi A, Kiyoshima K, Inokuchi J, Tatsugami K, Naito S. Risk factors for febrile neutropenia in patients receiving docetaxel chemotherapy for castration-resistant prostate cancer. Support Care Cancer. 2014;22(12):3219–26. https://doi.org/10.1007/s00520-014-2328-7.
Morgans AK, Chen Y-H, Sweeney CJ, Jarrard DF, Plimack ER, Gartrell BA, Carducci MA, Hussain M, Garcia JA, Cella D, Dipaola RS, Patrick-Miller LJ. Quality of life during treatment with chemohormonal therapy: analysis of E3805 chemohormonal androgen ablation randomized trial in prostate cancer. J Clin Oncol. 2018;36(11):1088–95. https://doi.org/10.1200/jco.2017.75.3335.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, Coskinas X, Frydenberg M, Hague WE, Horvath LG, Joshua AM, Lawrence NJ, Marx G, McCaffrey J, McDermott R, McJannett M, North SA, Parnis F, Parulekar W, Pook DW, Reaume MN, Sandhu SK, Tan A, Tan TH, Thomson A, Tu E, Vera-Badillo F, Williams SG, Yip S, Zhang AY, Zielinski RR, Sweeney CJ. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121–31. https://doi.org/10.1056/nejmoa1903835.
Chi KN, Agarwal N, Bjartell A, Chung BH, De Santana P, Gomes AJ, Given R, Juárez Soto Á, Merseburger AS, Özgüroğlu M, Uemura H, Ye D, Deprince K, Naini V, Li J, Cheng S, Yu MK, Zhang K, Larsen JS, McCarthy S, Chowdhury S. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13–24. https://doi.org/10.1056/nejmoa1903307.
Naito S, Tsukamoto T, Koga H, Harabayashi T, Sumiyoshi Y, Hoshi S, Akaza H. Docetaxel plus prednisolone for the treatment of metastatic hormone-refractory prostate cancer: a multicenter Phase II trial in Japan. Jpn J Clin Oncol. 2008;38(5):365–72. https://doi.org/10.1093/jjco/hyn029.
Narita S, Nara T, Kanda S, Numakura K, Saito M, Inoue T, Satoh S, Nanjo H, Tsuchiya N, Mitsuzuka K, Koie T, Kawamura S, Ohyama C, Tochigi T, Arai Y, Habuchi T. Radical prostatectomy with and without neoadjuvant chemohormonal pretreatment for high-risk localized prostate cancer: a comparative propensity score matched analysis. Clin Genitourin Cancer. 2019;17(1):e113–22. https://doi.org/10.1016/j.clgc.2018.09.019.
Acknowledgements
We would like to thank to Yoko Mitobe, Yukiko Sugiyama, Masako Nagata, and Saeko Nakamura for their assistance in performing this study.
Funding
This study was supported in part by a research grant from the MEXT/JSPS (Kakenhi, Nos. 16H02679, 19K18551, 19K16706, 19K09663). The funding bodies listed here do not have any roles in the design of the study or collection, analysis, and interpretation of data or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
YM, SN: Data collection, statistical analysis, manuscript writing. SH, SM, SC, KK, YA, RI, YT, ST, KN, NH, HS, AK, RI: Data collection. KO, TI, YH, TK, SA, JS, TS: protocol development. SN: Data analysis. CO: supervision. TH: manuscript editing, supervision. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
SN received honoraria from Janssen Pharmaceutical K.K., Bayer AG. Dr., AstraZeneca K.K., Takeda Pharmaceutical Company Ltd., Sanofi S.A., Astellas Pharma Inc. and grants for research from Novartis Pharmaceuticals.CO received honoraria from Janssen Pharmaceutical K.K., Takeda Pharmaceutical Company Ltd., Astellas Pharma Inc., Sanofi S.A., Bayer AG. and Nippon Shinyaku, Company Ltd. TH received honoraria from Janssen Pharmaceutical K.K., Takeda Pharmaceutical Company Ltd., Astellas Pharma Inc., Daiichi Sankyo Company, Ltd., AstraZeneca K.K.,Sanofi S.A., and Bayer AG. All the other authors declare that they have no conflicts of interest associated with this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muto, Y., Narita, S., Hatakeyama, S. et al. Short-term outcomes of risk-adapted upfront docetaxel administration in patients with metastatic hormone-sensitive prostate cancer: a multicenter prospective study in Japan. Med Oncol 38, 37 (2021). https://doi.org/10.1007/s12032-021-01480-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-021-01480-3