Abstract
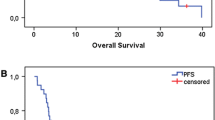
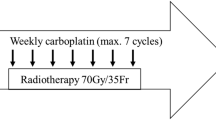
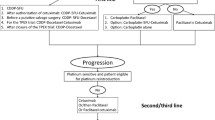
In locally advanced head and neck squamous-cell carcinoma (LA-HNSCC), clinical complete response (cCR) at the primary site, assessed by clinical examination, after induction chemotherapy predicts for a low relapse risk after subsequent chemoradiotherapy. Prior studies showed a cCR rate of 77% with induction nanoparticle albumin-bound (nab)-paclitaxel given with cisplatin and 5-fluorouracil (APF). The primary aims of this non-randomized phase 2 trial were to determine the cCR rate after induction nab-paclitaxel and cisplatin (Arm 1) and after nab-paclitaxel monotherapy (Arm 2). Eligibility required LA-HNSCC, T2-T4 stage classification, and suitable (Arm 1) or unsuitable (Arm 2) candidates for cisplatin. Arm 1 patients received nab-paclitaxel and cisplatin, then cisplatin with radiation. Arm 2 patients received nab-paclitaxel, then cetuximab with radiation. The primary endpoint was cCR after two cycles of induction chemotherapy. Each arm enrolled forty patients. cCR at the primary site occurred in 28 patients (70%) after nab-paclitaxel and cisplatin and in 8 patients (20%) after nab-paclitaxel monotherapy. The overall clinical response rate was 98% after nab-paclitaxel and cisplatin and 90% after nab-paclitaxel monotherapy. In subset analyses, cCR rates by T stage classifications (T2, T3, T4) were 54, 86, and 69% after nab-paclitaxel and cisplatin, and 14, 11, and 26% after nab-paclitaxel. cCR rates by human papillomavirus status (p16 positive oropharynx vs other) were 72 and 64% after nab-paclitaxel and cisplatin and 35 and 9% after nab-paclitaxel. The cCR rate after nab-paclitaxel and cisplatin was similar to APF; however, the cCR rate after nab-paclitaxel monotherapy was lower. The trial was registered at ClinicalTrials.gov NCT02573493 on October 9, 2015.




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143–54.
Ahn MJ, D’Cruz A, Vermoken JB, et al. Clinical recommendations for defining platinum unsuitable head and neck cancer patient populations on chemoradiotherapy: a literature review. Oral Oncol. 2016;53:10–6.
Baxi SS, O’Neill C, Sherman EJ, et al. Trends in chemoradiation use in elderly patients with head and neck cancer: changing treatment patterns with cetuximab. Head Neck. 2016;38:E165–71.
Pak K, Cheon GJ, Nam HY, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med. 2014;55:884–90.
Ghi MG, Paccagnella A, Ferrari D, et al. Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer: a phase II-III trial. Ann Oncol. 2017;28(9):2206–12.
Cohen EW, Karrison TG, Kocherginsky M, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32(25):2735–43.
Geoffrois L, Martin L, De Raucourt D, et al. Induction chemotherapy followed by cetuximab radiotherapy is not superior to concurrent chemoradiotherapy for head and neck carcinomas: results of the GORTEC 2007–02 phase III randomized trial. J Clin Oncol. 2018;36(31):3077–83.
Haddad R, O’Neill A, Rabinowits G, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013;14(3):257–64.
Recouvreux MV, Commisso C. Macropinocytosis: a metabolic adaptation to nutrient stress in cancer. Front Endocrinol. 2017;8:261.
Commisso C, Davidson SM, Soydaner-Azeloglu RD, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–8.
Nakase I, Kobayashi NB, Takatani-Nakase T, Yoshida T. Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Sci Rep. 2015;5:10300.
The Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–82.
Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12(4):1317–24.
Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–803.
Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055–62.
Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–703.
Hersh EM, Del Vecchio M, Brown MP, et al. A randomized, controlled phase III trial of nab-paclitaxel versus dacarbazine in chemotherapy-naïve patients with metastatic melanoma. Ann Oncol. 2015;26(11):2267–74.
Adkins D, Ley J, Trinkaus K, et al. A phase 2 trial of induction nab-paclitaxel and cetuximab given with cisplatin and 5-fluorouracil followed by concurrent cisplatin and radiation for locally advanced squamous cell carcinoma of the head and neck. Cancer. 2013;119(4):766–73.
Adkins D, Ley J, Michel L, et al. nab-Paclitaxel, cisplatin, and 5-fluorouracil followed by concurrent cisplatin and radiation for head and neck squamous cell carcinoma. Oral Oncol. 2016;61:1–7.
Adkins D, Ley J, Oppelt P, et al. nab-Paclitaxel-based induction chemotherapy with or without cetuximab for locally advanced head and neck squamous cell carcinoma. Oral Oncol. 2017;72:26–31.
Weiss J, Gilbert J, Deal AM, et al. Induction chemotherapy with carboplatin, nab-paclitaxel and cetuximab for at least N2b nodal status or surgically unresectable squamous cell carcinoma of the head and neck. Oral Oncol. 2018;84:46–51.
Seiwert TY, Foster CC, Blair EA, et al. OPTIMA: a phase II dose and volume de-escalation trial for human papillomavirus-positive oropharyngeal cancer. Ann Oncol. 2019;30(2):297–302.
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl. 1):122S-S150.
Ensley JF, Jacobs JR, Weaver A, et al. Correlation between response to cisplatinum-combination chemotherapy and subsequent radiotherapy in previously untreated patients with advanced squamous cell cancers of the head and neck. Cancer. 1984;54(5):811–4.
Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–8.
Hitt R, Lopez-Pousa A, Martinez-Trufero J, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol. 2005;23(34):8636–45.
Marur S, Li S, Cmelak AJ, et al. E1308: phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx—ECOG-ACRIN Cancer Research Group. J Clin Oncol. 2017;35(5):490–7.
Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393(10166):40–50.
Nguyen-Tan PF, Zhang Q, Ang KK, et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: Long-term report of efficacy and toxicity. J Clin Oncol. 2014;32(34):3858–67.
Rosenthal DI, Harari PM, Giralt J, et al. Association of human papillomavirus and p16 status with outcomes in the IMCL-9815 phase III registration trial for patients with locoregionally advanced oropharyngeal squamous cell carcinoma of the head and neck treated with radiotherapy with or without cetuximab. J Clin Oncol. 2016;34(12):1300–8.
Soliman HH. nab-Paclitaxel as a potential partner with checkpoint inhibitors in solid tumors. Onco Targets Ther. 2017;10:101–12.
Acknowledgements
The Siteman Cancer Center is supported in part by NCI Cancer Center Support (Grant number P30 CA91842). We acknowledge funding from Celgene for this project and the support of Tin Jin Ong MD, Kathy Amiri PhD, Jeff Eskew PhD, Monserrat Valles PhD, Jacqueline Jesse PharmD, and Daniel Read PhD. We thank the patients who participated, the head and neck research team at Washington University, Kim Trinkaus PhD, the Alvin Siteman Cancer Center (Washington University, St Louis, Missouri, USA) and Barnes-Jewish Hospital (St Louis) for use of the Tissue Procurement Center, and the Center for Biomedical Informatics.
Funding
This work was supported in part by Grant funding from Celgene. Clinical trial sponsorship by Celgene and Siteman Cancer Center in part by NCI Cancer Center Support Award Number P30 CA91842.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by JL, JL, and DA. The first draft of the manuscript was written by JL and DA, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
JL, MD, JR, RP, PP, JL, HG, PN, and MG have no disclosures to report. PO reports personal fees from Bristol Myers, Merck, and Eisai, outside the submitted work. RJ reports personal fees from Intuitive surgical, outside the submitted work. KP reports personal fees from Merck, Genetech, and Boehringer Ingelheim, outside the submitted work. RS reports consulting support from Bristol Myers and institutional research support from Merck, Bristol Myers, Pfizer, Vyriad, Incyte, Genentech, Actuate, and Novartis, outside the submitted work. WS reports consulting support from Bristol Myers and Regeneron, outside the submitted work. JZ reports personal fees from Summit Biolabs, Inc, outside the submitted work. WT reports other from Elekta, Inc (wife is employee. Radiotherapy hardware and software company. However, the department does not use those products). DA reports research funding from Celgene for the submitted work and consulting or scientific advisory board support from Pfizer, Eli Lilly, Merck, Celgene, Cue Biopharma, and institutional research support from Pfizer, Eli Lilly, Merck, Celgene/BMS, Novartis, AstraZeneca, Atara Bio, Blueprint Medicine, Celldex, Enzychem, Kura, Exelixis, Innate, Sensei, and Matrix Biomed, outside the submitted work.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. The study was reviewed and approved by the Washington University School of Medicine Internal Review Board, St. Louis, Missouri. Patients signed informed consent regarding publishing their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oppelt, P., Ley, J., Daly, M. et al. nab-Paclitaxel and cisplatin followed by cisplatin and radiation (Arm 1) and nab-paclitaxel followed by cetuximab and radiation (Arm 2) for locally advanced head and neck squamous-cell carcinoma: a multicenter, non-randomized phase 2 trial. Med Oncol 38, 35 (2021). https://doi.org/10.1007/s12032-021-01479-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-021-01479-w