Abstract
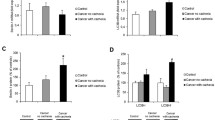
The incidence of gastric cancer cachexia is high and the clinical management is poor, so the study aimed to clarify the mechanism of muscle wasting to better screen patients with gastric cancer cachexia. Gastric cancer patients undergoing radical gastrectomy were divided into cachexia with sarcopenia (CS, n = 13) and normal (N, n = 10) two groups. The possible mechanism of skeletal muscle reduction was explored through Tandem Mass Tag (TMT) technique, Perls staining, Western blot analysis and measurement of oxidative stress indicators. The preoperative weight, weight loss, body mass index, calorie intake and skeletal muscle index values of the CS group were significantly lower than those of the N group (P < 0.05). We identified 114 differentially expressed proteins (DEP) in the muscles of two groups using TMT analysis. Bioinformatics analysis of DEP revealed that ferritin, iron and oxidative stress may be related to skeletal muscle consumption. Following Perls staining and measurement iron concentration in skeletal muscles, we found that the iron in the muscles of the CS group was significantly increased, and at the same time, western blot analysis showed that the expression of ferritin in the CS group was significantly increased and regulated by hepcidin-ferroportin axis. Finally, the CS group showed increased oxidative stress and weakened antioxidant stress systems in the muscles compared with the N group when oxidative stress indicators were analyzed. In conclusion, iron overload may be related to muscle loss in patients with gastric cancer cachexia. Gastric cancer patients with elevated ferritin are more likely to have muscle wasting.





Similar content being viewed by others
References
Yang L, Zheng R, Wang N, Yuan Y, Liu S, Li H, et al. Incidence and mortality of stomach cancer in China, 2014. Chin J Cancer Res. 2018;30:291–8.
Digklia A, Wagner AD. Advanced gastric cancer: current treatment landscape and future perspectives. World J Gastroenterol. 2016;22:2403–14.
Sun L, Quan XQ, Yu S. An epidemiological survey of cachexia in advanced cancer patients and analysis on its diagnostic and treatment status. Nutr Cancer. 2015;67:1056–62.
Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med. 2011;62:265–79.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95.
Zhuang C-L, Huang D-D, Pang W-Y, Zhou C-J, Wang S-L, Lou N, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer. Medicine. 2016;95:e3164.
Kudou K, Saeki H, Nakashima Y, Edahiro K, Korehisa S, Taniguchi D, et al. Prognostic significance of sarcopenia in patients with esophagogastric junction cancer or upper gastric cancer. Ann Surg Oncol. 2017;24:1804–10.
Mertins P, Tang LC, Krug K, Clark DJ, Gritsenko MA, Chen L, et al. Reproducible workflow for multiplexed deep-scale proteome and phosphoproteome analysis of tumor tissues by liquid chromatography-mass spectrometry. Nat Protoc. 2018;13:1632–61.
Dev S, Babitt JL. Overview of iron metabolism in health and disease. Hemodial Int. 2017;21(Suppl 1):S6-s20.
Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63:131–40.
Zhang Y, Wang J, Wang X, Gao T, Tian H, Zhou D, et al. The autophagic-lysosomal and ubiquitin proteasome systems are simultaneously activated in the skeletal muscle of gastric cancer patients with cachexia. Am J Clin Nutr. 2020;111:570–9.
Fleming RE, Ponka P. Iron overload in human disease. N Engl J Med. 2012;366:348–59.
Anderson GJ, Frazer DM. Current understanding of iron homeostasis. Am J Clin Nutr. 2017;106:1559s-s1566.
Altun M, Edstrom E, Spooner E, Flores-Moralez A, Bergman E, Tollet-Egnell P, et al. Iron load and redox stress in skeletal muscle of aged rats. Muscle Nerve. 2007;36:223–33.
Reardon TF, Allen DG. Iron injections in mice increase skeletal muscle iron content, induce oxidative stress and reduce exercise performance. Exp Physiol. 2009;94:720–30.
Ikeda Y, Imao M, Satoh A, Watanabe H, Hamano H, Horinouchi Y, et al. Iron-induced skeletal muscle atrophy involves an Akt-forkhead box O3–E3 ubiquitin ligase-dependent pathway. J Trace Elem Med Biol. 2016;35:66–76.
Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J, et al. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet. 2005;37:1264–9.
Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3.
Du F, Qian ZM, Gong Q, Zhu ZJ, Lu L, Ke Y. The iron regulatory hormone hepcidin inhibits expression of iron release as well as iron uptake proteins in J774 cells. J Nutr Biochem. 2012;23:1694–700.
Gu Z, Wang H, Xia J, Yang Y, Jin Z, Xu H, et al. Decreased ferroportin promotes myeloma cell growth and osteoclast differentiation. Cancer Res. 2015;75:2211–21.
Xue D, Zhou CX, Shi YB, Lu H, He XZ. Decreased expression of ferroportin in prostate cancer. Oncol Lett. 2015;10:913–6.
Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013;13:342–55.
Wang Y, Yu L, Ding J, Chen Y. Iron metabolism in cancer. Int J Mol Sci. 2018;20:95.
Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta. 1996;1275:161–203.
Rucker P, Torti FM, Torti SV. Role of H and L subunits in mouse ferritin. J Biol Chem. 1996;271:33352–7.
Kim TH, Hwang HJ, Kim SH. Relationship between serum ferritin levels and sarcopenia in Korean females aged 60 years and older using the fourth Korea National Health and Nutrition Examination Survey (KNHANES IV-2, 3), 2008–2009. PLoS ONE. 2014;9:e90105.
Nakagawa C, Inaba M, Ishimura E, Yamakawa T, Shoji S, Okuno S. Association of increased serum ferritin with impaired muscle strength/quality in hemodialysis patients. J Ren Nutr. 2016;26:253–7.
Oberley LW, Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979;39:1141–9.
Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–5.
Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30:1–12.
Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem. 2017;524:13–30.
Funding
This study was supported by National Natural Science Foundation of China (Grant No. 81770531) and Jiangsu Outstanding Youth Foundation (Grant No. BK20170009).
Author information
Authors and Affiliations
Contributions
WXY and ZY designed the study. GM, WSL, GXJ, and ZL collected the samples. ZD performed the experiments. ZD and ZY analyzed the data and wrote the manuscript. WXY and LGL reviewed/edited the manuscript. WXY and LGL supervised the research. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
There are no potential conflict of interest.
Research involved in human rights
The study was approved by the Ethics Committee of the Jinling hospital (Approval No. 2017NZKY-003-01).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, D., Zhang, Y., Mamtawla, G. et al. Iron overload is related to muscle wasting in patients with cachexia of gastric cancer: using quantitative proteome analysis. Med Oncol 37, 113 (2020). https://doi.org/10.1007/s12032-020-01439-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-020-01439-w