Abstract
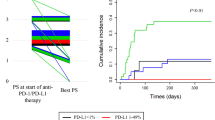
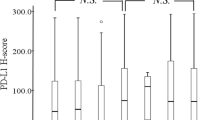
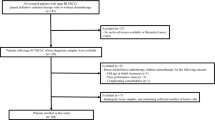
In non-small cell lung cancer (NSCLC) patients, the expression of tumor programmed death ligand 1 (PD-L1) is an important parameter for deciding the timing of the use of anti-programmed cell death 1 (PD-1) antibody. There has been increasing concern over the benefit of anti-PD-1 antibody in high-risk patients, such as those with preexisting interstitial lung disease (ILD). However, the status and value of PD-L1 as a predictive biomarker and the efficacy of anti-PD-1 antibody in NSCLC patients with preexisting ILD remains uncertain. We retrospectively reviewed the medical records of advanced/recurrent NSCLC patients who had undergone analysis of the tumor PD-L1 expression. We identified 358 patients with advanced/recurrent NSCLC who had undergone analysis of tumor PD-LI expression. Of these, 210 received anti-PD-1 antibody. Tumor-cell PD-L1 expression was similar between the groups with and without preexisting ILD (median, 35%; interquartile range, 0–70%; vs. median, 10%; interquartile range, 1–68%; p = 0.66). Of the 210 patients who received anti-PD-1 antibody, 14 patients had preexisting ILD. The progression-free survival (PFS) showed no significant difference between the patients receiving anti-PD-1 antibody with and without preexisting ILD (median PFS, 4.3 vs. 5.3 months; HR, 0.97; p = 0.84). Within the patients with preexisting ILD, the PFS was tended to be longer in the patients with tumor PD-L1 expression ≥ 50% than in those with tumor PD-L1 expression < 50% (median PFS, 12.5 vs. 2.5 months; HR, 0.47; p = 0.14). The value of PD-L1 expression as a biomarker may be comparable between patients with and without preexisting ILD.



Similar content being viewed by others
Abbreviations
- PD-1:
-
Programmed cell death 1
- PD-L1:
-
Programmed death ligand 1
- NSCLC:
-
Non-small cell lung cancer
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- ILD:
-
Interstitial lung disease
- RECIST:
-
Response evaluation criteria in solid tumors
- CT:
-
Computed tomography
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- EGFR:
-
Epidermal growth factor receptor
- ORR:
-
Overall response rate
- DCR:
-
Disease control rate
References
Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, Poddubskaya E, Borghaei H, Felip E, Paz-Ares L, Pluzanski A, Reckamp KL, Burgio MA, Kohlhaeufl M, Waterhouse D, Barlesi F, Antonia S, Arrieta O, Fayette J, Crino L, Rizvi N, Reck M, Hellmann MD, Geese WJ, Li A, Blackwood-Chirchir A, Healey D, Brahmer J, Eberhardt WEE. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, Phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924–33.
Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–35.
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39.
Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, Molina J, Kim J-H, Arvis CD, Ahn M-J, Majem M, Fidler MJ, de Castro G, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. The Lancet. 2016;387(10027):1540–50.
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee J-S, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. The Lancet. 2017;389(10066):255–65.
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, Investigators K-. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33.
King TE Jr. Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med. 2005;172(3):268–79.
Le Jeune I, Gribbin J, West J, Smith C, Cullinan P, Hubbard R. The incidence of cancer in patients with idiopathic pulmonary fibrosis and sarcoidosis in the UK. Respir Med. 2007;101(12):2534–40.
Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med. 2000;61(1):5–8.
Omori T, Tajiri M, Baba T, Ogura T, Iwasawa T, Okudela K, Takemura T, Oba MS, Maehara T, Nakayama H, Tsuboi M, Masuda M. Pulmonary resection for lung cancer in patients with idiopathic interstitial Pneumonia. Ann Thorac Surg. 2015;100(3):954–60.
Fujimoto D, Kato R, Morimoto T, Shimizu R, Sato Y, Kogo M, Ito J, Teraoka S, Nagata K, Nakagawa A, Otsuka K, Tomii K. Characteristics and prognostic impact of pneumonitis during systemic anti-cancer therapy in patients with advanced non-small-cell lung cancer. PLoS ONE. 2016;11(12):e0168465.
Fujimoto D, Sato Y, Morimoto T, Uehara K, Ito M, Otsuka K, Nagata K, Sakanoue I, Hamakawa H, Nakagawa A, Takahashi Y, Imai Y, Tomii K. Programmed cell death ligand 1 expression in non-small-cell lung cancer patients with interstitial lung disease: a matched case-control study. Clin Lung Cancer. 2018;19(5):e667–73.
Igawa S, Sato Y, Ryuge S, Ichinoe M, Katono K, Hiyoshi Y, Otani S, Nagashio R, Nakashima H, Katagiri M, Sasaki J, Murakumo Y, Satoh Y, Masuda N. Impact of PD-L1 expression in patients with surgically resected non-small-cell lung cancer. Oncology. 2017;92(5):283–90.
Shimoji M, Shimizu S, Sato K, Suda K, Kobayashi Y, Tomizawa K, Takemoto T, Mitsudomi T. Clinical and pathologic features of lung cancer expressing programmed cell death ligand 1 (PD-L1). Lung Cancer. 2016;98:69–75.
Fujimoto D, Tomii K, Otoshi T, Kawamura T, Tamai K, Takeshita J, Tanaka K, Matsumoto T, Monden K, Nagata K, Otsuka K, Nakagawa A, Hata A, Tachikawa R, Otsuka K, Hamakawa H, Katakami N, Takahashi Y, Imai Y. Preexisting interstitial lung disease is inversely correlated to tumor epidermal growth factor receptor mutation in patients with lung adenocarcinoma. Lung Cancer. 2013;80(2):159–64.
Mizushima Y, Kobayashi M. Clinical characteristics of synchronous multiple lung cancer associated with idiopathic pulmonary fibrosis. A review of Japanese cases. Chest. 1995;108(5):1272–7.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, Investigators K-. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28.
Kenmotsu H, Naito T, Kimura M, Ono A, Shukuya T, Nakamura Y, Tsuya A, Kaira K, Murakami H, Takahashi T, Endo M, Yamamoto N. The risk of cytotoxic chemotherapy-related exacerbation of interstitial lung disease with lung cancer. J Thorac Oncol. 2011;6(7):1242–6.
Ando M, Okamoto I, Yamamoto N, Takeda K, Tamura K, Seto T, Ariyoshi Y, Fukuoka M. Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol. 2006;24(16):2549–56.
Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, Rosenberg J, Voss M, Rudin CM, Rizvi H, Hou X, Rodriguez K, Albano M, Gordon RA, Leduc C, Rekhtman N, Harris B, Menzies AM, Guminski AD, Carlino MS, Kong BY, Wolchok JD, Postow MA, Long GV, Hellmann MD. Pneumonitis in patients treated with anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol. 2016;35(7):709.
Nishino M, Ramaiya NH, Awad MM, Sholl LM, Maattala JA, Taibi M, Hatabu H, Ott PA, Armand PF, Hodi FS. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22(24):6051–60.
Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, Sznol M, Long GV, Li H, Waxman IM, Jiang J, Robert C. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35(7):785–92.
Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, Massard C, Fuerea A, Ribrag V, Gazzah A, Armand JP, Amellal N, Angevin E, Noel N, Boutros C, Mateus C, Robert C, Soria JC, Marabelle A, Lambotte O. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–48.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SM has served on speakers’ bureaus for Taiho Pharmaceutical and Ono Pharmaceutical. YG has had consulting/advisory roles for Taiho Pharmaceutical; has served on speakers’ bureaus for Taiho Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb, MSD; and has received research funding from Taiho Pharmaceutical, Bristol-Myers Squibb, and Ono Pharmaceutical. SK has received research funding from Ono Pharmaceutical and has received honoraria from Ono Pharmaceutical, and Bristol-Myers Squibb. HH has received research funding from MSD, Bristol-Myers Squibb, Ono Pharmaceutical, and Taiho Pharmaceutical. YF has received research funding from MSD and has served on speakers’ bureaus from MSD, Taiho Pharmaceutical, Bristol-Myers Squibb, and Ono Pharmaceutical. NY has had consulting/advisory roles for Taiho Pharmaceutical; has served on speakers’ bureaus for Ono Pharmaceutical, Bristol-Myers Squibb, MSD; and has received honoraria from Ono Pharmaceutical, and MSD. NY has received research funding from Taiho Pharmaceutical, Bristol-Myers Squibb, and Ono Pharmaceutical and has served on speakers’ bureaus from Bristol-Myers Squibb, and Ono Pharmaceutical. YO has received research funding and honoraria from Taiho Pharmaceutical, MSD. All remaining authors have declared no conflict of interest.
Ethical approval
This study was approved by National Cancer Center Hospital Japan and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This study involved retrospective analysis of existing data with no patient intervention or interaction.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shibaki, R., Murakami, S., Matsumoto, Y. et al. Tumor expression and usefulness as a biomarker of programmed death ligand 1 in advanced non-small cell lung cancer patients with preexisting interstitial lung disease. Med Oncol 36, 49 (2019). https://doi.org/10.1007/s12032-019-1274-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-019-1274-0