Abstract
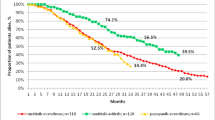
We aimed to compare oncological outcomes and safety of axitinib and sunitinib in patients with treatment-naïve metastatic renal cell carcinoma (mRCC). We retrospectively evaluated 169 patients with mRCC who were treated with axitinib or sunitinib as the first-line therapy in five hospitals between October 2008 and August 2018. Oncological outcomes and safety were compared between axitinib (n = 68) and sunitinib (n = 101) groups. Inverse probability of treatment weighted (IPTW)-adjusted Cox regression analysis was performed to evaluate effects of first-line therapies on progression-free survival (PFS), cancer-specific survival (CSS), and overall survival (OS). Patients in the axitinib group were significantly older (66 vs. 72 years) than those in the sunitinib group. Median relative dose intensity was significantly higher in the axitinib group (94 ± 62%) than in the sunitinib group (65 ± 20%; P = 0.001). Objective response rate was significantly higher in the axitinib group (21%) than in the sunitinib group (10%; P = 0.042). IPTW-adjusted Cox regression analysis revealed significant differences in CSS and OS but not in PFS between the two groups. Safety in terms of grade ≥ 3 adverse events was significantly different between the axitinib (34%) and sunitinib (55%) groups (P = 0.006). Compared with sunitinib, axitinib significantly prolonged CSS and OS and showed a safer profile as the first-line therapy for treatment-naïve mRCC.




Similar content being viewed by others
Abbreviations
- mRCC:
-
Metastatic renal cell carcinoma
- TKIs:
-
Tyrosine kinase inhibitors
- VEGF:
-
Vascular endothelial growth factor
- ECOG PS:
-
Eastern Cooperative Oncology Group performance status
- IMDC:
-
International Metastatic Renal Cell Carcinoma Database Consortium
- CR:
-
Complete response
- PR:
-
Partial response
- SD:
-
Stable disease
- PD:
-
Progressive disease
- PFS:
-
Progression-free survival
- CSS:
-
Cancer-specific survival
- OS:
-
Overall survival
- IPTW:
-
Inverse probability of treatment weighted
- HR:
-
Hazard ratio
- 95% CI:
-
95% confidence interval
- IQR:
-
Interquartile range
- RDI:
-
Relative dose intensity
- mTORi:
-
Mammalian target of rapamycin inhibitor
References
Hutson TE, Lesovoy V, Al-Shukri S, Stus VP, Lipatov ON, Bair AH, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol. 2013;14(13):1287–94. https://doi.org/10.1016/s1470-2045(13)70465-0.
Hutson TE, Al-Shukri S, Stus VP, Lipatov ON, Shparyk Y, Bair AH, et al. Axitinib versus sorafenib in first-line metastatic renal cell carcinoma: overall survival from a randomized phase III trial. Clin Genitourin Cancer. 2017;15(1):72–6. https://doi.org/10.1016/j.clgc.2016.05.008.
Pal SK, Signorovitch JE, Li N, Zichlin ML, Liu Z, Ghate SR, et al. Patterns of care among patients receiving sequential targeted therapies for advanced renal cell carcinoma: a retrospective chart review in the USA. Int J Urol. 2017;24(4):272–8. https://doi.org/10.1111/iju.13314.
Shinohara N, Abe T. Prognostic factors and risk classifications for patients with metastatic renal cell carcinoma. Int J Urol. 2015;22(10):888–97. https://doi.org/10.1111/iju.12858.
Tomita Y. Treatment strategies for advanced renal cell carcinoma: a new paradigm for surgical treatment. Int J Urol. 2016;23(1):13–21. https://doi.org/10.1111/iju.12899.
Lee JL, Kim MK, Park I, Ahn JH, Lee DH, Ryoo HM, et al. RandomizEd phase II trial of sunitinib four weeks on and two weeks off versus two weeks on and one week off in metastatic clear-cell type REnal cell carcinoma: restore trial. Ann Oncol. 2015;26(11):2300–5. https://doi.org/10.1093/annonc/mdv357.
Iwamoto K, Ishihara H, Takagi T, Kondo T, Yoshida K, Iizuka J, et al. Evaluation of relative dose intensity during the early phase of first-line sunitinib treatment using a 2-week-on/1-week-off regimen for metastatic renal cell carcinoma. Med Oncol. 2018;35(6):78. https://doi.org/10.1007/s12032-018-1139-y.
Kawashima A, Uemura M, Kato T, Ujike T, Nagahara A, Fujita K, et al. Results of weekday-on and weekend-off administration schedule of sunitinib therapy for advanced renal cell carcinoma. Int J Clin Oncol. 2018. https://doi.org/10.1007/s10147-018-1332-1.
Mouillet G, Paillard MJ, Maurina T, Vernerey D, Nguyen Tan Hon T, Almotlak H, et al. Open-label, randomized multicentre phase II study to assess the efficacy and tolerability of sunitinib by dose administration regimen (dose modification or dose interruptions) in patients with advanced or metastatic renal cell carcinoma: study protocol of the SURF trial. Trials. 2018;19(1):221. https://doi.org/10.1186/s13063-018-2613-8.
Guida FM, Santoni M, Conti A, Burattini L, Savini A, Zeppola T, et al. Alternative dosing schedules for sunitinib as a treatment of patients with metastatic renal cell carcinoma. Crit Rev Oncol Hematol. 2014;92(3):208–17. https://doi.org/10.1016/j.critrevonc.2014.07.006.
Kondo T, Takagi T, Kobayashi H, Iizuka J, Nozaki T, Hashimoto Y, et al. Superior tolerability of altered dosing schedule of sunitinib with 2-weeks-on and 1-week-off in patients with metastatic renal cell carcinoma–comparison to standard dosing schedule of 4-weeks-on and 2-weeks-off. Jpn J Clin Oncol. 2014;44(3):270–7. https://doi.org/10.1093/jjco/hyt232.
Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–9. https://doi.org/10.1016/s0140-6736(11)61613-9.
Ambring A, Bjorholt I, Lesen E, Stierner U, Oden A. Treatment with sorafenib and sunitinib in renal cell cancer: a Swedish register-based study. Med Oncol. 2013;30(1):331. https://doi.org/10.1007/s12032-012-0331-8.
Eichelberg C, Vervenne WL, De Santis M, Fischer von Weikersthal L, Goebell PJ, Lerchenmuller C, et al. SWITCH: a randomised, sequential, open-label study to evaluate the efficacy and safety of sorafenib-sunitinib versus sunitinib-sorafenib in the Treatment of Metastatic Renal Cell Cancer. Eur Urol. 2015;68(5):837–47. https://doi.org/10.1016/j.eururo.2015.04.017.
Oya M, Tomita Y, Fukasawa S, Shinohara N, Habuchi T, Rini BI, et al. Overall survival of first-line axitinib in metastatic renal cell carcinoma: Japanese subgroup analysis from phase II study. Cancer Sci. 2017;108(6):1231–9. https://doi.org/10.1111/cas.13232.
Horiguchi H, Yoneyama T, Hatakeyama S, Tokui N, Sato T, Fujita N, et al. Impact of bacillus Calmette-Guerin therapy of upper urinary tract carcinoma in situ: comparison of oncological outcomes with radical nephroureterectomy. Med Oncol. 2018;35(4):41. https://doi.org/10.1007/s12032-018-1102-y.
Kido K, Hatakeyama S, Fujita N, Yamamoto H, Tobisawa Y, Yoneyama T, et al. Oncologic outcomes for open and laparoscopic radical nephroureterectomy in patients with upper tract urothelial carcinoma. Int J Clin Oncol. 2018. https://doi.org/10.1007/s10147-018-1248-9.
Momota M, Hatakeyama S, Tokui N, Sato T, Yamamoto H, Tobisawa Y, et al. The impact of preoperative severe renal insufficiency on poor postsurgical oncological prognosis in patients with urothelial carcinoma. Eur Urol Focus. 2018. https://doi.org/10.1016/j.euf.2018.03.003.
Hamano I, Hatakeyama S, Iwamurau H, Fujita N, Fukushi K, Narita T, et al. Preoperative chronic kidney disease predicts poor oncological outcomes after radical cystectomy in patients with muscle-invasive bladder cancer. Oncotarget. 2017;8(37):61404–14. https://doi.org/10.18632/oncotarget.18248.
Hosogoe S, Hatakeyama S, Kusaka A, Hamano I, Iwamura H, Fujita N, et al. Platinum-based neoadjuvant chemotherapy improves oncological outcomes in patients with locally advanced upper tract urothelial carcinoma. Eur Urol Focus. 2017:231–40. https://doi.org/10.1016/j.euf.2017.03.013.
Kodama H, Hatakeyama S, Fujita N, Iwamura H, Anan G, Fukushi K, et al. Preoperative chronic kidney disease predicts poor oncological outcomes after radical nephroureterectomy in patients with upper urinary tract urothelial carcinoma. Oncotarget. 2017;8(47):83183–94. https://doi.org/10.18632/oncotarget.20554.
Kubota Y, Hatakeyama S, Tanaka T, Fujita N, Iwamura H, Mikami J, et al. Oncological outcomes of neoadjuvant chemotherapy in patients with locally advanced upper tract urothelial carcinoma: a multicenter study. Oncotarget. 2017;8(60):101500–8. https://doi.org/10.18632/oncotarget.21551.
Kusaka A, Hatakeyama S, Hosogoe S, Hamano I, Iwamura H, Fujita N, et al. Detecting asymptomatic recurrence after radical cystectomy contributes to better prognosis in patients with muscle-invasive bladder cancer. Med Oncol. 2017;34(5):90. https://doi.org/10.1007/s12032-017-0955-9.
Koie T, Ohyama C, Okamoto A, Yamamoto H, Imai A, Hatakeyama S, et al. Presurgical therapy with axitinib for advanced renal cell carcinoma: a case report. BMC Res Notes. 2013;6:484. https://doi.org/10.1186/1756-0500-6-484.
Koie T, Ohyama C, Yoneyama T, Yamamoto H, Imai A, Hatakeyama S, et al. Feasibly of axitinib as first-line therapy for advanced or metastatic renal cell carcinoma: a single-institution experience in Japan. BMC Urol. 2015;15:32. https://doi.org/10.1186/s12894-015-0027-4.
Sheng X, Bi F, Ren X, Cheng Y, Wang J, Rosbrook B, et al. First-line axitinib versus sorafenib in Asian patients with metastatic renal cell carcinoma: exploratory subgroup analyses of Phase III data. Future Oncol. 2018. https://doi.org/10.2217/fon-2018-0442.
Tanaka Y, Hatakeyama S, Hosogoe S, Tanaka T, Hamano I, Kusaka A, et al. Presurgical axitinib therapy increases fibrotic reactions within tumor thrombus in renal cell carcinoma with thrombus extending to the inferior vena cava. Int J Clin Oncol. 2018;23(1):134–41. https://doi.org/10.1007/s10147-017-1169-z.
Hosogoe S, Hatakeyama S, Kusaka A, Hamano I, Tanaka Y, Hagiwara K, et al. Contrast media enhancement reduction predicts tumor response to presurgical molecular-targeting therapy in patients with advanced renal cell carcinoma. Oncotarget. 2017. https://doi.org/10.18632/oncotarget.17930.
Kim KH, Kim HY, Kim HR, Sun JM, Lim HY, Lee HJ, et al. Efficacy and toxicity of sunitinib in patients with metastatic renal cell carcinoma with renal insufficiency. Eur J Cancer. 2014;50(4):746–52. https://doi.org/10.1016/j.ejca.2013.11.029.
Choueiri TK, Larkin J, Oya M, Thistlethwaite F, Martignoni M, Nathan P, et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol. 2018;19(4):451–60. https://doi.org/10.1016/s1470-2045(18)30107-4.
Acknowledgements
The authors would like to thank Takuma Narita, Teppei Okamoto, Itsuto Hamano, Hirotaka Horiguchi, Masaaki Oikawa, Daisuke Noro, Kazuhisa Hagiwara, Yuki Fujita, Yukie Nishizawa, and Satomi Sakamoto for their invaluable support in data collection. The authors would also like to thank Enago (http://www.enago.jp) for English language review.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (Nos. 17K11118, 17K11119, 17K16768, 17K16770, 17K167711, 18K16681, 18K16682, 18K16717, 18K16718, 18K16719, and 18K09157) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Rights and permissions
About this article
Cite this article
Konishi, S., Hatakeyama, S., Tanaka, T. et al. Comparison of axitinib and sunitinib as first-line therapies for metastatic renal cell carcinoma: a real-world multicenter analysis. Med Oncol 36, 6 (2019). https://doi.org/10.1007/s12032-018-1231-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-018-1231-3