Abstract
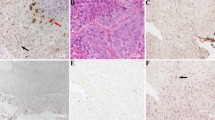
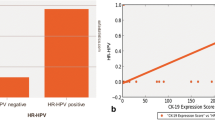
Human papillomavirus (HPV) infection modulates several host cytokines contributing to cancer development. Oncostatin M (OSM), an IL-6 family cytokine, acts to promote cell senescence and inhibit growth. Its dysregulation promotes cell survival, cell proliferation and metastasis in various malignancies. The effect of HPV on OSM dysregulation has not been investigated. To elucidate this, immunohistochemistry was used on formalin-fixed, paraffin-embedded oral squamous cell carcinoma (OSCC) tissues: HPV-positive (50) and HPV-negative (50) cases. Immortalized human cervical keratinocytes expressing HPV16E6 (HCK1T, Tet-On system) were used to demonstrate the role of HPV16E6 in OSM expression. In addition, a vector containing HPV16E6/E7 was transiently transfected into oral cancer cell lines. Cell viability, cell-cycle progression and cell migration were evaluated using flow cytometry and a wound healing assay, respectively. The results showed various intensities of OSM expression in OSCC. Interestingly, the median percentages of strongly stained cells were significantly higher in HPV-positive OSCCs than in HPV-negative OSCCs. To explore the role of HPV oncoproteins on OSM expression, the expression of HPV16E6 in the HCK1T Tet-On condition was induced by doxycycline and HPV16E6 was found to significantly upregulate levels of OSM mRNA and protein, with concomitant upregulation of c-Myc. In addition, the levels of OSM mRNA and protein in E6/E7 transiently transfected oral cancer cells also gradually increased in a time-dependent manner and these transfected cells showed greater viability and higher migration rates and cell-cycle progression than controls. This result demonstrates that HPV16 oncoproteins upregulate OSM and play an important role to promote OSCC development.






Similar content being viewed by others
Abbreviations
- Dox:
-
Doxycycline
- FFPE:
-
Formalin-fixed, paraffin-embedded
- HCK1T:
-
Immortalized human cervical keratinocyte
- HPV:
-
Human papillomavirus
- IHC:
-
Immunohistochemistry
- ISH:
-
In situ hybridization
- OSCC:
-
Oral squamous cell carcinoma
- OSM:
-
Oncostatin M
- TCA:
-
Trichloroacetic acid
- TMA:
-
Tissue microarray
References
Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917. doi:10.1002/ijc.25516.
Zhang B, Li P, Wang E, Brahmi Z, Dunn KW, Blum JS, et al. The E5 protein of human papillomavirus type 16 perturbs MHC class II antigen maturation in human foreskin keratinocytes treated with interferon-γ. Virology. 2003;310(1):100–8. doi:10.1016/S0042-6822(03)00103-X.
Amador-Molina A, Hernández-Valencia JF, Lamoyi E, Contreras-Paredes A, Lizano M. Role of innate immunity against human papillomavirus (HPV) infections and effect of adjuvants in promoting specific immune response. Viruses. 2013;5(11):2624–42. doi:10.3390/v5112624.
Ren C, Cheng X, Lu B, Yang G. Activation of interleukin-6/signal transducer and activator of transcription 3 by human papillomavirus early proteins 6 induces fibroblast senescence to promote cervical tumourigenesis through autocrine and paracrine pathways in tumour microenvironment. Eur J Cancer. 2013;49(18):3889–99. doi:10.1016/j.ejca.2013.07.140.
Chang Y-H, Yu C-W, Lai L-C, Tsao C-H, Ho K-T, Yang S-C, et al. Up-regulation of interleukin-17 expression by human papillomavirus type 16 E6 in nonsmall cell lung cancer. Cancer. 2010;116(20):4800–9. doi:10.1002/cncr.25224.
Scaffidi AK, Mutsaers SE, Moodley YP, McAnulty RJ, Laurent GJ, Thompson PJ, et al. Oncostatin M stimulates proliferation, induces collagen production and inhibits apoptosis of human lung fibroblasts. Br J Pharmacol. 2002;136(5):793–801. doi:10.1038/sj.bjp.0704769.
Canady J, Arndt S, Karrer S, Bosserhoff AK. Increased KGF expression promotes fibroblast activation in a double paracrine manner resulting in cutaneous fibrosis. J Invest Dermatol. 2013;133(3):647–57. doi:10.1038/jid.2012.389.
Ryan RE, Martin B, Mellor L, Jacob RB, Tawara K, McDougal OM, et al. Oncostatin M binds to extracellular matrix in a bioactive conformation: implications for inflammation and metastasis. Cytokine. 2015;72(1):71–85. doi:10.1016/j.cyto.2014.11.007.
Junk DJ, Bryson BL, Jackson MW. HiJAK’d signaling; the STAT3 paradox in senescence and cancer progression. Cancers. 2014;6(2):741–55. doi:10.3390/cancers6020741.
Mercader M, Taddeo B, Panella JR, Chandran B, Nickoloff BJ, Foreman KE. Induction of HHV-8 lytic cycle replication by inflammatory cytokines produced by HIV-1-infected T cells. Am J Pathol. 2000;156(6):1961–71. doi:10.1016/S0002-9440(10)65069-9.
James MA, Lee JH, Klingelhutz AJ. Human papillomavirus type 16 E6 activates NF-κB, induces cIAP-2 expression, and protects against apoptosis in a PDZ binding motif-dependent manner. J Virol. 2006;80(11):5301–7. doi:10.1128/JVI.01942-05.
Bernard C, Merval R, Lebret M, Delerive P, Dusanter-Fourt I, Lehoux S, et al. Oncostatin M Induces interleukin-6 and cyclooxygenase-2 expression in human vascular smooth muscle cells synergy with interleukin-1β. Circ Res. 1999;85(12):1124–31. doi:10.1161/01.RES.85.12.1124.
David E, Tirode F, Baud’huin M, Guihard P, Laud K, Delattre O, et al. Oncostatin M is a growth factor for Ewing sarcoma. Am J Pathol. 2012;181(5):1782–95. doi:10.1016/j.ajpath.2012.07.023.
Kan CE, Cipriano R, Jackson MW. c-MYC functions as a molecular switch to alter the response of human mammary epithelial cells to oncostatin M. Cancer Res. 2011;71(22):6930–9. doi:10.1158/0008-5472.CAN-10-3860.
García-Tuñón I, Ricote M, Ruiz A, Fraile B, Paniagua R, Royuela M. OSM, LIF, its receptors, and its relationship with the malignance in human breast carcinoma (in situ and in infiltrative). Cancer Invest. 2008;26(3):222–9. doi:10.1080/07357900701638491.
Su C-M, Lee W-L, Hsu C-J, Lu T-T, Wang L-H, Xu G-H, et al. Adiponectin induces oncostatin M expression in osteoblasts through the PI3 K/Akt signaling pathway. Int J Mol Sci. 2015;17(1):29. doi:10.3390/ijms17010029.
Tummers B, Van Der Burg SH. High-risk human papillomavirus targets crossroads in immune signaling. Viruses. 2015;7(5):2485–506. doi:10.3390/v7052485.
Tran T-A, Ross JS, Sheehan CE, Carlson JA. Comparison of oncostatin M expression in keratoacanthoma and squamous cell carcinoma. Mod Pathol. 2000;13(4):427–32. doi:10.1038/modpathol.3880073.
Guo L, Chen C, Shi M, Wang F, Chen X, Diao D, et al. Stat3-coordinated Lin-28–let-7–HMGA2 and miR-200–ZEB1 circuits initiate and maintain oncostatin M-driven epithelial–mesenchymal transition. Oncogene. 2013;32(45):5272–82. doi:10.1038/onc.2012.573.
Tsuchiya N, Izumiya M, Ogata-Kawata H, Okamoto K, Fujiwara Y, Nakai M, et al. Tumor suppressor miR-22 determines p53-dependent cellular fate through post-transcriptional regulation of p21. Cancer Res. 2011;71(13):4628–39. doi:10.1158/0008-5472.
Wang X, Wang H-K, Li Y, Hafner M, Banerjee NS, Tang S, et al. microRNAs are biomarkers of oncogenic human papillomavirus infections. Proc Natl Acad Sci USA. 2014;111(11):4262–7. doi:10.1073/pnas.1401430111.
Acknowledgments
This project was supported by Grant of Faculty of Medicine, Khon Kaen University (Grant Number IN59117), and Khon Kaen University, Thailand (Grant Number 581202). We would like to acknowledge Prof. David Blair, for editing the MS via Publication Clinic KKU, Thailand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval was given by the Khon Kaen University Ethics Committee (No. HE 581211). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study, giving their authorization to access their clinical information and tumor samples for research purpose.
Rights and permissions
About this article
Cite this article
Chuerduangphui, J., Pientong, C., Chaiyarit, P. et al. Effect of human papillomavirus 16 oncoproteins on oncostatin M upregulation in oral squamous cell carcinoma. Med Oncol 33, 83 (2016). https://doi.org/10.1007/s12032-016-0800-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-016-0800-6