Abstract
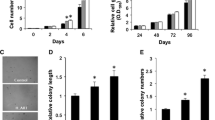
Both tumor suppressor and tumor promoter roles, which are dependent on the tumor type, have been described for caveolin-1 (CAV-1). Because CAV-1 can modulate cell signaling, we tested the hypothesis that it regulates lung adenocarcinoma cell proliferation and metastasis via modulation of epidermal growth factor receptor (EGFR) activity. The lung adenocarcinoma cell line, GLC-82, was transfected with pcDNA3.1CAV-1 plasmid, before cell proliferation, migration, and invasion were analyzed. In the in vivo xenograft model, the relationship between the CAV-1 expression and EGFR phosphorylation and signaling was assessed by western blot analysis. The relationship between the CAV-1 as well as Ki67 expression and the clinicopathological characteristics of 68 lung adenocarcinoma patients was also examined using immunohistochemistry. Overexpression of CAV-1 significantly increased GLC-82 proliferation (p < 0.001), migration (p < 0.001), and invasion (p = 0.002) as well as EGFR and ERK phosphorylation (p < 0.05). The GLC-82/CAV-1 cell tumors were also significantly larger than those of control cells (all p ≤ 0.05). In lung adenocarcinoma patients, CAV-1 expression was positively correlated with lymph node metastasis and cancer stage. Finally, CAV-1 expression was associated with the expression of Ki-67, a marker of cell proliferation. CAV-1 enhanced GLC-82 cell proliferation, migration, and invasion possibly through EGFR and ERK signaling. Furthermore, the relationship of CAV-1 with Ki67 expression, a marker of proliferative capacity, in lung adenocarcinoma samples is suggestive of its role in disease progression. Further studies are required to confirm the role of CAV-1 in the metastasis of lung adenocarcinoma as well as its potential prognostic and therapeutic value.








Similar content being viewed by others
Change history
29 September 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12032-022-01807-8
References
Ahannan SV, Brahmer JR. Antiangiogenic agents in combination with chemotherapy in patients with advanced non-small cell lung cancer. Cancer Invest. 2011;29:325–37.
Stinchcombe TE. Recent advances in the treatment of non-small cell and small cell lung cancer. F1000Prime Rep. 2014;6:117.
Filosto S, Becker CR, Goldkorn T. Cigarette smoke induces aberrant EGF receptor activation that mediates lung cancer development and resistance to tyrosine kinase inhibitors. Mol Cancer Ther. 2012;11:795–804.
Luetterforst R, Stang E, Zorzi N, Carozzi A, Way M, Parton RG. Molecular characterization of caveolin association with the Golgi complex: identification of a cis-Golgi targeting domain in the caveolin molecule. J Cell Bio. 1999;145:1443–59.
Glenney JR, Soppet D. Sequence and expression of caveolin, a protein component of caveolae plasma-membrane domains phosphorylated on tyrosine in Rous sarcoma virus-transformed fibroblasts. Proc Natl Acad Sci USA. 1992;89:10517–21.
Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288(3):494–506.
Couet J, Sargiacomo M, Lisanti MP. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem. 1997;272:30429–38.
Engelman JA, Chu C, Lin A, Jo H, Ikezu T, Okamoto T, et al. Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett. 1995;428:205–11.
Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, et al. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem. 1995;270:15693–701.
Razani B, Rubin CS, Lisanti MP. Regulation of cAMP mediated signal transduction via interaction of caveolins with the catalytic subunit of protein kinase A. J Biol Chem. 1999;274:26353–60.
Conde MC, Ramirez-Lorca R, Lopez-Jamar JM, Molero E, Ramírez-Armengol JA, Moreno Nogueira JA, et al. Genetic analysis of Caveolin-1 and eNOS genes in colorectal cancer. Oncol Rep. 2006;16:353–9.
Bouras T, Lisanti MP, Pestell RG. Caveolin-1 in breast cancer. Cancer Biol. 2004;3:931–41.
Kato T, Miyamoto M, Kato K, Cho Y, Itoh T, Morikawa T, et al. Difference of caveolin-1 expression pattern in human lung neoplastic tissue. Atypical adenomatous hyperplasia, adenocarcinoma and squamous cell carcinoma. Cancer Lett. 2004;214(1):121–8.
Bender FC, Reymond MA, Bron C, Quest AF. Caveolin-1 levels are down-regulated in human colon tumors, and ectopic expression of caveolin-1 in colon carcinoma cell lines reduces cell tumorigenicity. Cancer Res. 2000;60:5870–8.
Wiechen K, Diatchenko L, Agoulnik A, Scharff KM, Schober H, Arlt K, et al. Caveolin–1 is downregulated in human ovarian carcinoma and acts as a candidate tumor suppressor gene. Am J Pathol. 2001;159:1635–40.
Razani BA, Itschuler Y, Zhu L, Pestell RG, Mostov KE, Lisanti MP. Caveolin-1 expression is downregulated in cells transformed by the human papilloma virus in a P53-dependent manner replacement of Caveolin-1 expression suppresses HPV-mediated cell transformation. Biochemisty. 2000;39(45):13916–24.
Yang G, Truong LD, Wheeler TM, Thompson TC. Caveolin-1 expression in clinically confined human prostate cancer: a novel prognostic marker. Cancer Res. 1999;59:5719–23.
Horiguchi A, Asano T, Asakuma J, Asano T, Sumitomo M, Hayakawa M. Impact of caveolin-1 expression on clinicopathological parameters in renal cell carcinoma. J Urol. 2004;172:718–22.
Fong A, Garcia E, Gwynn L, Lisanti MP, Fazzari MJ, Li M. Expression of caveolin-1 and caveolin-2 in urothelial carcinoma of the urinary bladder correlates with tumor grade and squamous differentiation. J Am J Clin Pathol. 2003;120:93–100.
Chen D, Shen C, Du H, Zhou Y, Che G. Duplex value of caveolin-1 in non-small cell lung cancer: a meta analysis. Fam Cancer. 2014;13:449–57.
Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118.
Morgillo F, Martinelli E, Troiani T, Orditura M, De Vita F, Ciardiello F. Antitumor activity of sorafenib in human cancer cell lines with acquired resistance to EGFR and VEGFR tyrosine kinase inhibitors. PLoS ONE. 2011;6:e28841.
Yi WY, Hong W, Kang HJ, Kim HJ, Zhao W, Wang A, et al. Inhibition of the PI3 K/AKT pathway potentiates cytotoxicity of EGFR kinase inhibitors in triple-negative breast cancer cells. J Cell Mol Med. 2013;17:648–56.
Filaria JL, Zhu TN, O’Connell MP, Hoek KS, Indig FE, Frank BP, et al. Filamin A modulates kinase activation and intracellular trafficking of epidermal growth factor receptors in human melanoma cells. Endocrinology. 2009;150:2551–60.
Pancotti F, Roncuzzi L, Maggiolini M, Gasperi-Campani A. Caveolin-1 silencing arrests the proliferation of metastatic lung cancer cells through the inhibition of STAT3 signaling. Cell Signal. 2007;24:1390–7.
Ha TK, Her NG, Lee MG, Ryu BK, Lee JH, Han J, et al. Caveolin-1 increases aerobic glycolysis in colorectal cancers by stimulating HMGA1-mediated GLUT3 transcription. Cancer Res. 2012;72:4097–109.
Linge A, Weinhold K, Bläsche R, Kasper M, Barth K. Downregulation of caveolin-1 affects bleomycin-induced growth arrest and cellular senescence in A549 cells. Int J Biochem Cell Biol. 2007;39:1964–74.
Han F, Zhang J, Shao J, Yi X. Caveolin-1 promotes an invasive phenotype and predicts poor prognosis in large cell lung carcinoma. Pathol Res Pract. 2014;210:514–20.
Cassoni P, Daniele L, Maldi E, Righi L, Tavaglione V, Novello S, et al. Caveolin-1 expression in lung carcinoma varies according to tumour histotype and is acquired de novo in brain metastases. Histopathology. 2009;55:20–7.
Wang Y, Liu B, Xu Y, Zhang J, Xia Q, Yu B, et al. Correlation of caveolin-1 expression with clinicopathologic features and prognosis in patients with lung adenocarcinoma. Chinese Journal of Pathology. 2014;43:251–5.
Ho CC, Huang PH, Huang HY, Chen YH, Yang PC, Hsu SM. Up-regulated caveolin-1 accentuates the metastasis capability of lung adenocarcinoma by inducing filopodia formation. Am J Pathol. 2002;61:1647–56.
Kirita K, Ishii G, Matsuwaki R, Matsumura Y, Umemura S, Matsumoto S, et al. Identification of biological properties of intralymphatic tumor related to the development of lymph node metastasis in lung adenocarcinoma. PLoS ONE. 2013;8:e83537.
Rendon BE, Roger T, Teneng I, Zhao M, Al-Abed Y, Calandra T, et al. Regulation of human lung adenocarcinoma cell migration and invasion by macrophage migration inhibitory factor. J Biol Chem. 2007;282:29910–8.
Chen HL, Fan LF, Gao J, Ouyang JP, Zhang YX. Differential expression and function of the caveolin-1 gene in non-small cell lung carcinoma. Oncol Rep. 2011;25:359–66.
Taron M, Ichinose Y, Rosell R, Mok T, Massuti B, Zamora L, et al. Activating mutations in the tyrosine kinase domain of the epidermal growth factor receptor are associated with improved survival in gefitinib-treated chemorefractory lung adenocarcinomas. Clin Cancer Res. 2005;11:5878–85.
Liu HX, Xing LX, Wang HB, Yang JQ, Sun YM. Relationship between expression of caveolin-1 and pERK1/2 and prognosis in non-small cell lung cancer. Chinese Journal of Pathology. 2008;37(9):615–9.
Zhan P, Shen XK, Qian Q, Wang Q, Zhu JP, Zhang Y, et al. Expression of caveolin-1 is correlated with disease stage and survival in lung adenocarcinomas. Oncol Rep. 2012;27(4):1072–8.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81071846, to T.N.Z); Natural Science Foundation of Hebei Province of China (No. H2013505059, to T.N.Z); Department of Science and Technology of Hebei Province of China (No. 12396107D, to R.J.Z, No. 09966114D, and No. 092461102D, to T.N.Z); Wu Jieping Foundation (No. 320.6750.12604, and No. 320.6750.14063, to T.N.Z).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Luan, TY., Zhu, TN., Cui, YJ. et al. Expression of caveolin-1 is correlated with lung adenocarcinoma proliferation, migration, and invasion. Med Oncol 32, 207 (2015). https://doi.org/10.1007/s12032-015-0644-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0644-5