Abstract
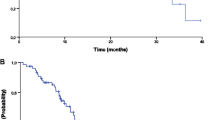
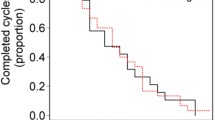
The aim of the study was to retrospectively assess the efficacy and safety of low-dose metronomic oral capecitabine in pretreated or frail patients with recurrent colorectal cancer. Patients with recurrent colorectal cancer and prior treatment with fluoropyrimidines, oxaliplatin, and irinotecan or unable to receive standard chemotherapy because of toxicity concerns were included. Treatment consisted of oral capecitabine 1,500 mg daily until disease progression or unacceptable toxicity. Response rates were determined according to RECIST criteria. The end points were disease control rate [(DCR) consisting of complete response, partial response (PR), and stable disease (SD)], overall survival (OS), and safety. Sixty-eight patients, median age 72.5 years, were treated. The median number of previous treatments was 2 (range 0–5). Sixty-two percent of patients had received ≥2 previous lines of treatment. The overall DCR was 26 %, PR in 2 (3 %) and SD in 14 (23 %). Nineteen percent of patients were progression free for at least 6 months. In an exploratory analysis, there was a significant relation of performance status with DCR (HR = 3.3; P = 0.05). The median OS was 8 months. DCR was associated with a longer survival (HR = 0.4; P < 0.01). Grade 3 toxicities included anemia (1), diarrhea (1), and hand-foot syndrome (1). There were no cases of grade 4 toxicity or treatment-related deaths. Metronomic capecitabine was moderately active and well-tolerated in pretreated or frail patients with recurrent colorectal cancer.

Similar content being viewed by others
References
Gasparini G. Metronomic scheduling: the future of chemotherapy? Lancet Oncol. 2001;2:733–40.
Browder T, Butterfield CE, Kräling BM, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–86.
Klement G, Baruchel S, Rak J, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumour regression without overt toxicity. J Clin Invest. 2000;105:R15–24.
Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–36.
Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumour angiogenesis in mice. J Clin Invest. 2000;105:1045–7.
Romiti A, Cox MC, Sarcina I, et al. Metronomic chemotherapy for cancer treatment: a decade of clinical studies. Cancer Chemother Pharmacol. 2013;72:13–33.
Colleoni M, Rocca A, Sandri MT, et al. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumour activity and correlation with vascular endothelial growth factor levels. Ann Oncol. 2002;13:73–80.
Allegrini G, Di Desidero T, Barletta MT, et al. Clinical, pharmacokinetic and pharmacodynamic evaluations of metronomic UFT and cyclophosphamide plus celecoxib in patients with advanced refractory gastrointestinal cancers. Angiogenesis. 2012;15:275–86.
Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4 + CD25 + regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8.
Generali D, Bates G, Berruti A, et al. Immunomodulation of FOXP3 + regulatory T cells by the aromatase inhibitor letrozole in breast cancer patients. Clin Cancer Res. 2009;15:1046–51.
Folkins C, Man S, Xu P, et al. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67:3560–4.
Lee K, Qian DZ, Rey S, et al. Anthracycline chemotherapy inhibits HIF-1 transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc Natl Acad Sci USA. 2009;106:2353–8.
Shi H, Jiang J, Ji J, et al. Anti-angiogenesis participates in antitumor effects of metronomic capecitabine on colon cancer. Cancer Lett. 2014;349:28–35.
Steinbild S, Arends J, Medinger M, et al. Metronomic antiangiogenic therapy with capecitabine and celecoxib in advanced tumor patients-results of a phase II study. Onkologie. 2007;30:629–35.
Lin PC, Chen WS, Chao TC, et al. Biweekly oxaliplatin plus 1-day infusional fluorouracil/leucovorin followed by metronomic chemotherapy with tegafur/uracil in pretreated metastatic colorectal cancer. Cancer Chemother Pharmacol. 2007;60:351–6.
Lara PM, Redman MW, Kelly K, et al. Disease control rate at 8 weeks predicts clinical benefit in advanced non-small-cell lung cancer: results from Southwest Oncology Group randomized trials. J Clin Oncol. 2008;26:463–7.
Miger J, Holmqvist A, Sun XF, et al. Low-dose capecitabine (Xeloda) for treatment for gastrointestinal cancer. Med Oncol. 2014;31:870.
Dellapasqua S, Bertolini F, Bagnardi V, et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol. 2008;26:4899–905.
Wong NS, Buckman RA, Clemons M, et al. Phase I/II trial of metronomic chemotherapy with daily dalteparin and cyclophosphamide, twice-weekly methotrexate, and daily prednisone as therapy for metastatic breast cancer using vascular endothelial growth factor and soluble vascular endothelial growth factor receptor levels as markers of response. J Clin Oncol. 2010;28:723–30.
Khan OA, Blann AD, Payne MJ, et al. Continuous low-dose cyclophosphamide and methotrexate combined with celecoxib for patients with advanced cancer. Br J Cancer. 2011;104:1822–7.
Di Rocco R, Romiti A, Simmaco M, et al. TSER and MTHFR gene polymorphisms and toxicity in cancer patients treated with fluoropyrimidine based chemotherapy. Ann Oncol. 2010;21(Suppl 6):viii248.
Rosmarin D, Palles C, Church D, et al. Genetic markers of toxicity from capecitabine and other fluorouracil-based regimens: investigation in the QUASAR2 study, systematic review, and meta-analysis. J Clin Oncol. 2014;32:1031–9.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romiti, A., Onesti, C.E., Roberto, M. et al. Continuous, low-dose capecitabine for patients with recurrent colorectal cancer. Med Oncol 32, 54 (2015). https://doi.org/10.1007/s12032-015-0496-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0496-z