Abstract
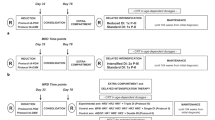
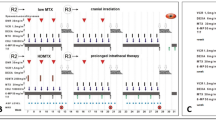
Clinical risk classification is inaccurate in predicting outcome in adult patients with acute lymphoblastic leukemia (ALL), sometimes resulting in patients receiving inappropriate chemotherapy or stem cell transplantation. To identify complementary markers suitable for further treatment stratification in patients with standard-risk (SR)/philadelphia-negative (Ph-negative) precursor B-ALL, we evaluated the predictive value of minimal residual disease (MRD) after induction and consolidation chemotherapy in strictly defined SR/Ph-negative precursor B-ALL patients who were treated with a standard protocol using quantitative real-time polymerase chain reaction with the rearranged immunoglobulin heavy chain gene as a molecular marker. The cytologic complete response (CR) rate was 92.3 % after induction. At this time point, the molecular CR rate was 73.9 %. Patients with molecular CR (MolCR) after induction had a significantly higher probability of disease-free survival (DFS; 78.8 vs 30.8 %; P = .001) and of overall survival (OS; 82.4 vs 41.7 %; P < .0001) compared to patients with molecular failure (MolFail). MRD at end consolidation had the same significance. Quantitative MRD assessment identified patients with MolFail after induction and/or consolidation as a high-risk group, with 3-year DFS and OS rates of 28.6 and 35.7 %, respectively. Patients with MolCR after induction and consolidation were classified as low-risk and had 3-year DFS rate of 89.7 % and OS rate of 93.3 %. Thus, MRD quantification during treatment identified prognostic subgroups within the otherwise homogeneous SR/Ph-negative precursor B-ALL population who may benefit from individualized treatment.



Similar content being viewed by others
References
Silverman LB, Gelber RD, Dalton VK, Asselin B, Barr R, Clavell L, et al. Approved outcome for children with acute lymphoblastic leukemia: results of Dana Farber Consortium protocol 91-01. Blood. 2001;97:1211–8.
Pui C-H, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–78.
Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM, Lazarus HM, Franklin IM, Litzow MR, Ciobanu N, et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106:3760–7.
Le QH, Thomas X, Ecochard R, Iwaz J, Lheritier V, Michallet M, Fiere D. Initial and late prognostic factors to predict survival in adult acute lymphoblastic leukaemia. Eur J Haematol. 2006;77:471–9.
Faderl S, O’Brien S, Pui CH, Stock W, Wetzler M, Hoelzer D, Kantarjian HM. Adult acute lymphoblastic leukemia: concepts and strategies. Cancer. 2010;116:1165–76.
Appelbaum FR, Rosenblum D, Arceci RJ, Carrol WL, Breitfeld P, Forman S, et al. End points to establish the efficacy of new agents in the treatment of acute leukemia. Blood. 2007;109(5):1810–6.
Szczepanski T. Why and how to quantify minimal residual disease in acute lymphoblastic leukemia? Leukemia. 2007;21(4):622–6.
Campana D. Role of minimal residual disease monitoring in adult and pediatric acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009;23(5):1083–98.
Dworzak MN, Froschl G, Printz D, Mann G, Potschger U, Muhlegger N, Fritsch G, Gadner H, Austrian Berlin–Frankfurt–Munster Study Group. Prognostic significance and modalities of flow cytometric minimal residual disease detection in childhood acute lymphoblastic leukemia. Blood. 2002;99(6):1952–8.
Nyvold C, Madsen O, Ryder LP, Seyfarth J, Svejgaard A, Clausen N, et al. Precise quantification of minimal residual disease at day 29 allows identification of children with acute lymphoblastic leukemia and an excellent outcome. Blood. 2002;99:1253–8.
Pongers-Willemse MJ, Verhagen OJ, Tibbe GJ, Wijkhuijs AJ, de Haas V, Roovers E, van der Schoot CE, van Dongen JJ. Real-time quantitative PCR for the detection of minimal residual disease in acute lymphoblastic leukemia using junctional region-specific TaqMan probes. Leukemia. 1998;12(12):2006–14.
Moppett J, Burke GA, Steward CG, Oakhill A, Goulden NJ. The clinical relevance of detection of minimal residual disease in childhood acute lymphoblastic leukaemia. J Clin Pathol. 2003;56(4):249–53.
Chang J, Medlin S, Kahl B, Longo W, Williams E, Lionberger J, Kim K, Kim J, Esterberg E, Juckett M. Augmented and standard Berlin-Frankfurt-Münster chemotherapy for treatment of adult acute lymphoblastic leukemia. Leuk Lymphoma. 2008;49(12):2298–307.
Brisco MJ, Hughes E, Neoh SH, Sykes P, Bradstock K, Enno A, et al. Relationship between minimal residual disease and outcome in adult acute lymphoblastic leukemia. Blood. 1996;87:5251–6.
Verhagen OJ, Willemse MJ, Breunis WB, Wijkhuijs AJ, Jacobs DC, Joosten SA, van Wering ER, van Dongen JJ, van der Schoot CE. Application of germline IGH probes in real-time quantitative PCR for the detection of minimal residual disease in acute lymphoblastic leukemia. Leukemia. 2000;14(8):1426–35.
Ram R, Gafter-Gvili A, Vidal L, Paul M, Ben-Bassat I, Shpilberg O, et al. Management of adult patients with acute lymphoblastic leukemia in first complete remission: systematic review and meta-analysis. Cancer. 2010;116(14):3447–57.
Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, Burnett AK, Chopra R, Wiernik PH, Foroni L, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111:1827–33.
Rowe JM, Buck G, Burnett AK, Burnett A, Chopra R, Wiernik P, et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood. 2005;106(12):3760–7.
Hoelzer D, Huettmann A, Kaul F, Irmer S, Jaekel N, Mohren M, et al. Immunochemotherapy with rituximab improves molecular CR-rate and outcome in CD20+ B-lineage standard and high-risk patients; results of 263 CD20+ patients studied prospectively in GMALL study 07/2003 [abstract]. Blood (ASH Annual Meeting Abstracts). 2010; 116 (21): Abstract 170.
Gökbuget N, Kneba M, Raff T, Trautmann H, Bartram C-R, Arnold R, Fietkau R, Freund M, Ganser A, Ludwig W-D, et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120:1868–76.
Bassan R, Spinelli O, Oldani E, Intermesoli T, Tosi M, Peruta B, et al. Improved risk classification for risk-specific therapy based on the molecular study of MRD in adult ALL. Blood. 2009;113(18):4153–62.
Vidriales MB, Perez JJ, Lopez-Berges MC, Gutie′rrez N, Ciudad J, Lucio P, Vazquez L, Garcı′a-Sanz R, del Cañizo MC, Ferna′ndez-Calvo J, Ramos F, Rodríguez MJ, Calmuntia MJ, Porwith A, Orfao A, San-Miguel JF. Minimal residual disease in adolescent (older than 14 years) and adult acute lymphoblastic leukemias: early immunophenotypic evaluation has high clinical value. Blood. 2003;101:4695–700.
Holowiecki J, Krawczyk-Kulis M, Giebel S, Jagoda K, Stella-Holowiecka B, Piatkowska-Jakubas B, et al. Status of minimal residual disease after induction predicts outcome in both standard and high-risk Ph-negative adult acute lymphoblastic leukemia. The Polish Adult Leukemia Group ALL 4-2002 MRD study. Br J Haematol. 2008;142(2):227–37.
Cimino G, Elia L, Rapanotti M, Sprovieri T, Mancini M, Cuneo A, Mecucci C, Fioritoni G, Carotenuto M, Morra E, Liso V, Annino L, Saglio G, De Rossi G, Foà R, Mandelli F. Transcript in patients with t(4;11) acute lymphoblastic leukemia A prospective study of residual-disease monitoring of theALL1/AF4. Blood. 2000;95:96–101.
Stock W, La M, Sanford B, Bloomfield CD, Vardiman JW, Gaynon P, Larson RA, Nachman J, Children’s Cancer G, Cancer, Leukemia Group Bs: What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children’s Cancer Group and Cancer and LeukemiaGroup B studies. Blood 2008;112:1646–54.
Huguet F, Leguay T, Raffoux E, Thomas X, Beldjord K, Delabesse E, Chevallier P, Buzyn A, Delannoy A, Chalandon Y, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol. 2009;27:911–8.
Schafer ES, Hunger SP. Optimal therapy for acute lymphoblastic leukemia in adolescents and young adults. Nat Rev Clin Oncol. 2011;8:417–24.
Vora A, Goulden N, Wade R, Mitchell C, Hancock J, Hough R, Rowntree C, Richards S. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14(3):199–209.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salah-Eldin, M., Abousamra, N.K. & Azzam, H. Clinical significance of minimal residual disease in young adults with standard-risk/Ph-negative precursor B-acute lymphoblastic leukemia: results of prospective study. Med Oncol 31, 938 (2014). https://doi.org/10.1007/s12032-014-0938-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0938-z