Abstract
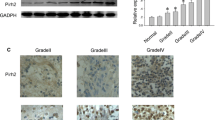
SYF2 is thought to be a cell cycle regulator at the G1/S transition, which encodes a nuclear protein that interacts with cyclin D-type binding-protein 1. In the present study, we investigated the role of SYF2 in human glioma progression. Immunohistochemical and Western blot analyses were performed in human glioma tissues. High SYF2 expression (located in cell nuclei) was observed in 80 samples, and its level was correlated with the grade of malignancy. A strongly positive correlation was observed between SYF2 and Ki-67 expression (P < 0.01). More importantly, high expression of SYF2 was associated with a poor outcome. In vitro, after the release of U87 cell lines from serum starvation, the expression of SYF2 was upregulated, as well as PCNA and cyclin D1. In addition, knockdown of SYF2 by small interfering RNA transfection diminished the expression of PCNA, cyclin D1 and arrested cell growth at G1 phase. These results indicate that SYF2 in glioma is essential for cell proliferation; thus, targeting SYF2 or its downstream targets may lead to novel therapies for glioblastomas.




Similar content being viewed by others
References
Assem M, Sibenaller Z, Agarwal S, Al-Keilani MS, Alqudah MA, Ryken TC. Enhancing diagnosis, prognosis, and therapeutic outcome prediction of gliomas using genomics. OMICS. 2012;16(3):113–22. doi:10.1089/omi.2011.0031.
Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: surveillance, epidemiology, and end results program, 1973 to 2001. Neurosurg Focus. 2006;20(4):E1. doi:10.3171/foc.2006.20.4.E1.
Reardon DA, Herndon JE 2nd, Peters KB, Desjardins A, Coan A, Lou E, et al. Bevacizumab continuation beyond initial bevacizumab progression among recurrent glioblastoma patients. Br J Cancer. 2012;107(9):1481–7. doi:10.1038/bjc.2012.415.
Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi:10.1056/NEJMra0708126.
Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15(11):1311–33. doi:10.1101/gad.891601.
Zhuang W, Qin Z, Liang Z. The role of autophagy in sensitizing malignant glioma cells to radiation therapy. Acta Biochim Biophys Sin. 2009;41(5):341–51.
Sherr CJ. Cancer cell cycles. Science. 1996;274(5293):1672–7.
Chang MS, Chang CL, Huang CJ, Yang YC. p29, a novel GCIP-interacting protein, localizes in the nucleus. Biochem Biophys Res Commun. 2000;279(2):732–7. doi:10.1006/bbrc.2000.3992.
Sherr CJ. Mammalian G1 cyclins. Cell. 1993;73(6):1059–65.
Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14(3):2077–86.
Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81(3):323–30.
Harbour JW, Dean DC. Rb function in cell-cycle regulation and apoptosis. Nat Cell Biol. 2000;2(4):E65–7. doi:10.1038/35008695.
Chang MS, Chen CY, Yeh HI, Fan CC, Huang CJ, Yang YC. Cloning, expression, and genomic organization of mouse mp29 gene. Biochem Biophys Res Commun. 2002;299(2):241–6.
Chu PC, Yang YC, Lu YT, Chen HT, Yu LC, Chang MS. Silencing of p29 affects DNA damage responses with UV irradiation. Cancer Res. 2006;66(17):8484–91. doi:10.1158/0008-5472.CAN-05-3229.
Chu PC, Wang TY, Lu YT, Chou CK, Yang YC, Chang MS. Involvement of p29 in DNA damage responses and Fanconi anemia pathway. Carcinogenesis. 2009;30(10):1710–6. doi:10.1093/carcin/bgp204.
Dahan O, Kupiec M. Mutations in genes of Saccharomyces cerevisiae encoding pre-mRNA splicing factors cause cell cycle arrest through activation of the spindle checkpoint. Nucleic Acids Res. 2002;30(20):4361–70.
Chen CH, Chu PC, Lee L, Lien HW, Lin TL, Fan CC, et al. Disruption of murine mp29/Syf2/Ntc31 gene results in embryonic lethality with aberrant checkpoint response. PLoS One. 2012;7(3):e33538. doi:10.1371/journal.pone.0033538.
Xu W, Cao M, Zheng H, Tan X, Li L, Cui G, et al. Upregulation of SYF2 is associated with neuronal apoptosis caused by reactive astrogliosis to neuroinflammation. J Neurosci Res. 2014;92(3):318–28. doi:10.1002/jnr.23312.
Huang X, Liu F, Zhu C, Cai J, Wang H, Wang X, et al. Suppression of KIF3B expression inhibits human hepatocellular carcinoma proliferation. Dig Dis Sci. 2014;59(4):795–806. doi:10.1007/s10620-013-2969-2.
Ding Z, Liu X, Liu Y, Zhang J, Huang X, Yang X, et al. Expression of far upstream element (FUSE) binding protein 1 in human glioma is correlated with c-Myc and cell proliferation. Mol Carcinog. 2013;. doi:10.1002/mc.22114.
Wang Y, Yang S, Ni Q, He S, Zhao Y, Yuan Q, et al. Overexpression of forkhead box J2 can decrease the migration of breast cancer cells. J Cell Biochem. 2012;113(8):2729–37. doi:10.1002/jcb.24146.
Ferguson SD. Malignant gliomas: diagnosis and treatment. Disease-a-Month. 2011;57(10):558–69. doi:10.1016/j.disamonth.2011.08.020.
Rampling R, James A, Papanastassiou V. The present and future management of malignant brain tumours: surgery, radiotherapy, chemotherapy. J Neurol Neurosurg Psychiatry. 2004;75(Suppl 2):ii24–30.
Qi S, Song Y, Peng Y, Wang H, Long H, Yu X, et al. ZEB2 mediates multiple pathways regulating cell proliferation, migration, invasion, and apoptosis in glioma. PLoS One. 2012;7(6):e38842. doi:10.1371/journal.pone.0038842.
Zhao B, Wang H, Zong G, Li P. The role of IFITM3 in the growth and migration of human glioma cells. BMC Neurol. 2013;13:210. doi:10.1186/1471-2377-13-210.
Vincent K, Wang Q, Jay S, Hobbs K, Rymond BC. Genetic interactions with CLF1 identify additional pre-mRNA splicing factors and a link between activators of yeast vesicular transport and splicing. Genetics. 2003;164(3):895–907.
Ma W, Stafford LJ, Li D, Luo J, Li X, Ning G, et al. GCIP/CCNDBP1, a helix–loop–helix protein, suppresses tumorigenesis. J Cell Biochem. 2007;100(6):1376–86. doi:10.1002/jcb.21140.
Witzel II, Koh LF, Perkins ND. Regulation of cyclin D1 gene expression. Biochem Soc Trans. 2010;38(Pt 1):217–22. doi:10.1042/BST0380217.
Acknowledgments
This work was supported by the China Natural Science Foundation (81201976, 81000963), Jiangsu Province’s Natural Science Foundation (BK2012670), Jiangsu Province’s Health Department (z201318), Yancheng Medical Science Development Foundation (YK2013003, YK2013019).
Conflict of interest
All authors have declared all sources of funding for the research reported in this manuscript and have no financial or other contractual agreements that might cause conflicts of interest or be perceived as causing conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jun Guo and Lixiang Yang have contributed equally in this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12032_2014_101_MOESM1_ESM.tif
Fig. S1 The negative control of immunohistochemical analyses. Negative control slides were processed in parallel using a nonspecific immunoglobulin IgG at the same concentration as the primary antibody
Rights and permissions
About this article
Cite this article
Guo, J., Yang, L., Huang, J. et al. Knocking down the expression of SYF2 inhibits the proliferation of glioma cells. Med Oncol 31, 101 (2014). https://doi.org/10.1007/s12032-014-0101-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0101-x