Abstract
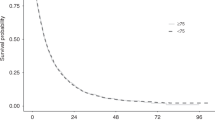
The incidence of hepatocellular carcinoma (HCC) is increasing worldwide and the proportion of older patients with HCC is expected to steadily rise in the next years. Sorafenib is the standard of care for patients with advanced HCC but there is a lack of detailed data on how older patients with cirrhosis tolerate this drug. Therefore, we aimed to evaluate the impact of age on the effects of sorafenib-targeted therapy in patients with HCC and cirrhosis. We analyzed a consecutive cohort of HCC patients not eligible for surgery or locoregional treatment, with Child-Pugh score ≤7, and an Eastern Cooperative Oncology Group performance status of 0–1, treated with sorafenib. Clinical outcomes and treatment-related adverse events (AEs) were compared between younger (<70 years) and older (≥70 years) patients. Overall, 150 patients, 90 in the younger (median age 60 years) and 60 in the older (median age 72 years) group, were evaluated. Treatment duration was 4 months in both groups. The median time to progression and overall survival were longer in older than in younger group (12 vs. 8 months and 16 vs. 12 months, respectively), although the differences did not reach a statistical significance. Grade 3–4 AEs were more frequently observed in younger than in older group (15.7 vs. 9.2 %, respectively; p = .0146). In field practice, sorafenib treatment in elderly patients with cirrhosis and HCC resulted at least as effective and safe as in younger patients. However, severe AEs occurred more frequently in younger patients.


Similar content being viewed by others
References
GLOBOCAN Cancer incidence and mortality worldwide. International Agency for Research on Cancer, World Health Organization, 2010. Available at http://globocan.iarc.fr (2008). Accessed 3 June 2011.
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76.
Benvegnù L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53:744–9.
Stroffolini T, Trevisani F, Pinzello G, Brunello F, Tommasini MA, Iavarone M, et al. Changing aetiological factors of hepatocellular carcinoma and their potential impact on the effectiveness of surveillance. Dig Liver Dis. 2011;43:875–80.
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;24(359):378–90.
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109.
Liu L, Cao Y, Chen C, McNabola A, Wilkie D, Wilhelm S, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–8.
Eisen T, Oudard S, Szczylik C, Gravis G, Heinzer H, Middleton R, et al. Sorafenib for older patients with renal cell carcinoma: subset analysis from a randomized trial. J Natl Cancer Inst. 2008;100:1454–63.
Bukowski RM, Stadler WM, McDermott DF, Figlin A, Knox JJ, Gabrail N, et al. Safety and efficacy of sorafenib in elderly patients treated in the North American advanced renal cell carcinoma sorafenib expanded access program. Oncology. 2010;78:340–7.
Pili R, Guo Y, Chang J, Nakanishi H, Martin GR, Passaniti A. Altered angiogenesis under lying age-dependent changes in tumor growth. J Natl Cancer Inst. 1994;86:1303–14.
Kaptzan T, Skutelsky E, Itzhaki O, et al. Efficacy of anti-angiogenic treatment of tumors in old versus young mice. Mech Ageing Dev. 2006;127:398–409.
Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European association for the study of the liver. J Hepatol. 2001;35:421–30.
Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–38.
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
Balducci L. Geriatric oncology: challenge for the new century. Eur J Cancer. 2000;36:1741–54.
Yancik R, Wesley MN, Ries LA, Havlik RJ, Long S, Edwards BK, Yates JW. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: a population-based study. Cancer. 1998;82:2123–34.
Sastre J, Marcuello E, Masutti B, et al. Irinotecan in combination with fluorouracilin a 48-hour continuous infusion as first-line chemotherapy for elderly patients with metastatic colorectal cancer: a Spanish cooperative group for the treatment of digestive tumors study. J Clin Oncol. 2005;23:3545–51.
Doherty JG, Rufer A, Bartholomew P, Beaumont DM. The presentation, treatment and outcome of renal cell carcinoma in old age. Age Ageing. 1999;28:359–62.
Balducci L, Extermann M. Management of cancer in the older person: a practical approach. Oncologist. 2000;5:224–37.
Kyriakou F, Kountourakis P, Papamichael D. Targeted agents: review of toxicity in the elderly metastatic colorectal cancer patients. Target Oncol. 2011;6:245–51.
El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37(Suppl 2):S88–94.
Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB, editors. Cancer incidence in five continents. Volume VIII. IARC Scientific Publications No. 155. Lyon, France: International Agency for Research on Cancer; International Association of Cancer Registries. 2002.
White LA, Menzin J, Korn JR, Friedman M, Lang K, Ray S. Medical care costs and survival associated with hepatocellular carcinoma among the elderly. Clin Gastroenterol Hepatol. 2012;10:547-54.
Mirici-Cappa F, Gramenzi A, Santi V, Zambruni A, Di Micoli A, Frigerio M, et al. Treatments for hepatocellular carcinoma in elderly patients are as effective as in younger patients: a 20-year multicentre experience. Gut. 2010;59:387–96.
Langer CJ. Clinical evidence on the undertreatment of older and poor performance patients who have advanced non-small-cell lung cancer: is there a role for targeted therapy in these cohorts? Clin Lung Cancer. 2011;12:272–9.
Larochelle P, Kollmannsberger C, Feldman RD, Schiffrin EL, Poirier L, Patenaude F, Ruether D, Myers M, Bjarnason G. Hypertension management in patients with renal cell cancer treated with anti-angiogenic agents. Curr Oncol. 2012;19:202–8.
Imarisio I, Paglino C, Ganini C, Magnani L, Caccialanza R, Porta C. The effect of sorafenib treatment on the diabetic status of patients with renal cell or hepatocellular carcinoma. Future Oncol. 2012;8:1051–7.
Wong H, Tang YF, Yao TJ, Chiu J, Leung R, Chan P, et al. The outcomes and safety of single-agent sorafenib in the treatment of elderly patients with advanced hepatocellular carcinoma. Oncologist. 2011;16:1721–8.
Morimoto M, Numata K, Kondo M, Hidaka H, Takada J, Shibuya A, et al. Higher discontinuation and lower survival rates are likely in elderly Japanese patients with advanced hepatocellular carcinoma receiving sorafenib. Hepatol Res. 2011;41:296–302.
Bellmunt J, Négrier S, Escudier B, Awada A. SIOG Taskforce. The medical treatment of metastatic renal cell cancer in the elderly: position paper of a SIOG Taskforce. Crit Rev Oncol Hematol. 2009;69:64–72.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Di Costanzo, G.G., Tortora, R., De Luca, M. et al. Impact of age on toxicity and efficacy of sorafenib-targeted therapy in cirrhotic patients with hepatocellular carcinoma. Med Oncol 30, 446 (2013). https://doi.org/10.1007/s12032-012-0446-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-012-0446-y