Abstract
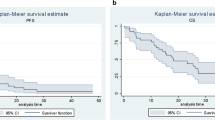
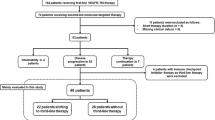
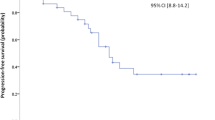
Sequential therapy is a standard strategy used to overcome the limitations of targeted agents in metastatic renal cell carcinoma. It remains unclear whether a mammalian target of rapamycin (mTOR) inhibitor is a more effective second-line therapy after first-line vascular endothelial growth factor tyrosine kinase inhibitor (VEGF TKI) has failed than the alternative, VEGF TKI. A clinical database was used to identify all patients with renal cell carcinoma who failed at first-line VEGF TKI and then treated with second-line VEGF TKI or mTOR inhibitors in the Asan Medical Center. Patient medical characteristics, radiological response and survival status were assessed. Of the 83 patients who met the inclusion criteria, 41 received second-line VEGF TKI [sunitinib (n = 16) and sorafenib (n = 25)] and 42 were treated with mTOR inhibitors [temsirolimus (n = 11) and everolimus (n = 31)]. After a median follow-up duration of 23.9 months (95 % CI, 17.8–30.0), progression-free survival was 3.0 months for both groups [hazard ratio (HR, VEGF TKI vs. mTOR inhibitor) = 0.97, 95 % CI 0.59–1.62, P = 0.92]. Overall survival was 10.6 months for the VEGF TKI group and 8.2 months for the mTOR inhibitor group (HR = 0.98, 95 % CI 0.57–1.68, P = 0.94). The two groups did not differ significantly in terms of disease control rate (51 % for VEGF TKI and 59 % for mTOR inhibitor, P = 0.75). Second-line VEGF TKI seems to be as effective as mTOR inhibitors and may be a viable option as a second-line agent after first-line anti-VEGF agents have failed.

Similar content being viewed by others
References
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–34.
Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370(9605):2103–11.
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–81.
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–56.
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–24.
Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26(33):5422–8.
Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–8.
Dudek AZ, Zolnierek J, Dham A, Lindgren BR, Szczylik C. Sequential therapy with sorafenib and sunitinib in renal cell carcinoma. Cancer. 2009;115(1):61–7.
Eichelberg C, Heuer R, Chun FK, Hinrichs K, Zacharias M, Huland H, et al. Sequential use of the tyrosine kinase inhibitors sorafenib and sunitinib in metastatic renal cell carcinoma: a retrospective outcome analysis. Eur Urol. 2008;54(6):1373–8.
Sablin MP, Negrier S, Ravaud A, Oudard S, Balleyguier C, Gautier J, et al. Sequential sorafenib and sunitinib for renal cell carcinoma. J Urol. 2009;182(1):29–34.
Di Lorenzo G, Carteni G, Autorino R, Bruni G, Tudini M, Rizzo M, et al. Phase II study of sorafenib in patients with sunitinib-refractory metastatic renal cell cancer. J Clin Oncol. 2009;27(27):4469–74.
Rini BI, Wilding G, Hudes G, Stadler WM, Kim S, Tarazi J, et al. Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27(27):4462–8.
Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–9.
Britten CD, Kabbinavar F, Hecht JR, Bello CL, Li J, Baum C, et al. A phase I and pharmacokinetic study of sunitinib administered daily for 2 weeks, followed by a 1-week off period. Cancer Chemother Pharmacol. 2008;61(3):515–24.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–9.
Patel PH, Senico PL, Curiel RE, Motzer RJ. Phase I study combining treatment with temsirolimus and sunitinib malate in patients with advanced renal cell carcinoma. Clin Genitourin Cancer. 2009;7(1):24–7.
Feldman DR, Baum MS, Ginsberg MS, Hassoun H, Flombaum CD, Velasco S, et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(9):1432–9.
Negrier S, Gravis G, Perol D, Chevreau C, Delva R, Bay JO, et al. Temsirolimus and bevacizumab, or sunitinib, or interferon alfa and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): a randomised phase 2 trial. Lancet Oncol. 2011;12(7):673–80.
Herrmann E, Marschner N, Grimm MO, Ohlmann CH, Hutzschenreuter U, Overkamp F, et al. Sequential therapies with sorafenib and sunitinib in advanced or metastatic renal cell carcinoma. World J Urol. 2011;29(3):361–6.
Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26(1):127–32.
Schmidinger M, Bellmunt J. Plethora of agents, plethora of targets, plethora of side effects in metastatic renal cell carcinoma. Cancer Treat Rev. 2010;36(5):416–24.
Heng DY, Mackenzie MJ, Vaishampayan UN, Bjarnason GA, Knox JJ, Tan MH, et al. Primary anti-vascular endothelial growth factor (VEGF)-refractory metastatic renal cell carcinoma: clinical characteristics, risk factors, and subsequent therapy. Ann Oncol. 2011;Nov 5:[Epub ahead of print].
Clinicalconnection. A randomized trial of temsirolimus versus sorafenib as second-line therapy in patients with advanced renal cell carcinoma who have failed first-line sunitinib therapy. Clinicalconnection. 2012. http://75.126.186.193/exp/EPVS.aspx?studyID=240312&slID=448904. Accessed 22 Nov 2011.
Acknowledgments
The study was supported by a grant from the Korean Health Technology R&D project, Ministry of Health & Welfare, Republic of Korea (Grant Number: A070001, A102059) and by the Covering Research Center Program, Ministry of Education, Science and Technology, Republic of Korea (Project Number: 2011K000871).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, K., Lee, JL., Park, I. et al. Comparative efficacy of vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor (TKI) and mammalian target of rapamycin (mTOR) inhibitor as second-line therapy in patients with metastatic renal cell carcinoma after the failure of first-line VEGF TKI. Med Oncol 29, 3291–3297 (2012). https://doi.org/10.1007/s12032-012-0227-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-012-0227-7