Abstract
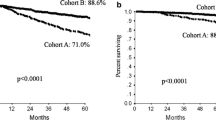
There are many controversies regarding the treatment of very early-stage (pT1a/bN0M0) breast cancer (BC), generally considered to have a very good prognosis. The debate is the benefit of an adjuvant treatment of HER2-neu (namely HER-2)-positive subcentimetric carcinoma with trastuzumab. Current guidelines do not suggest, with the highest level of evidence, whether trastuzumab should be administered after adjuvant chemotherapy in the treatment of high-risk pT1a/bN0M0 breast cancer. The major phase III trials that confirmed the benefit of adjuvant immunotherapy did not include small (<1 cm diameter) node-negative breast cancer. Several retrospective case series of HER-2-positive pT1a/bN0M0 carcinoma seem to demonstrate that they have a higher risk of relapse compared to the HER-2-negative counterpart. HER-2 also seems to confer an independent risk of recurrence and/or death in a multivariate analysis within large node-negative breast cancer populations. In particular, the best way to select higher-risk tumours that may achieve the best results from a trastuzumab-based therapy appears to be the in situ hybridization, which should follow the new recommended algorithm of the ASCO/CAP guidelines in case of doubtful results. According to the evidence that the survival of HER-2-positive BC can be improved with the introduction of trastuzumab respect to the HER-2-negative counterpart, there is today less uncertainty about the curative role of anti-HER-2 therapy in very early disease.
Similar content being viewed by others
References
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717.
Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al., editors. AJCC cancer staging handbook: TNM classification of malignant tumours. 6th ed. New York: Wiley; 2002.
http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf.
Mirza AN, Mirza NQ, Vlastos G, Singletary SE. Prognostic factors in node-negative breast cancer. Ann Surg. 2002;235(1):10–26.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82.
Rudolph P, Olsson H, Bonatz G, Ratjen V, Bolte H, Baldetorp B, et al. Correlation between p53, c-erbB-2, and topoisomerase IIa expression, DNA ploidy, hormonal receptor status and proliferation in 356 node negative breast carcinomas: prognostic implications. J Pathol. 1999;187:207–16.
Gasparini G, Gullick WJ, Maluta S, Dalla Palma P, Caffo O, Leonardi E, et al. c-erbB-3 and c-erbB-2 protein expression in node-negative breast carcinoma—an immunocytochemical study. Eur J Cancer. 1994;30A:16–22.
Quenel N, Wafflart J, Bonichon F, de Mascarel I, Trojani M, Durand M, et al. The prognostic value of c-erbB-2 in primary breast carcinomas: a study on 942 cases. Breast Cancer Res Treat. 1995;35:283–91.
Gusterson BA, Gelber RD, Goldhirsch A, Price KN, Save-Soderborgh J, Anbazhagan tR, et al. Prognostic importance of c-erbB-2 expression in breast cancer. J Clin Oncol. 1992;10:1049–56.
Bianchi S, Paglierani M, Zampi G, Cardona G, Cataliotti L, Bonardi R, et al. Prognostic significance of c-erbB-2 expression in node-negative breast cancer. Br J Cancer. 1993;67:625–9.
Tsutsui S, Ohno S, Murakami S, Hachitanda Y, Oda S. Prognostic value of c-ErbB2 expression in breast cancer. J Surg Oncol. 2002;72:216–23.
Press MF, Bernstein L, Thomas PA, Meisner LF, Zhou JY, Ma Y, et al. HER2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol. 1997;15:2894–904.
Choi YH, Ahn JH, Kim SB, Jung KH, Gong GY, Kim MJ, Son BH, Ahn SH, Kim WK. Tissue microarray-based study of patients with lymph node-negative breast cancer shows that HER2/neu over-expression is an important predictive marker of poor prognosis. Ann Oncol. 2009 [Epub ahead of print].
De Boer RH, Chan A. Clinical outcomes of patients with T1N0 breast cancers: how important are biological risk factors? Program and abstracts of the 31st annual San Antonio breast cancer symposium, 10–14 Dec 2008, San Antonio, Texas. Abstract 2078.
Mass RD, Press MF, Anderson S, Cobleigh MA, Vogel CL, Dybdal N, et al. Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer. 2005;6(3):240–6.
Schmidt M, Lewark B, Kohlschmidt N, Glawatz C, Steiner E, Tanner B, et al. Long-term prognostic significance of HER-2/neu in untreated node-negative breast cancer depends on the method of testing. Breast Cancer Res. 2005;7:R256–66.
Andrulis IL, Bull SB, Blackstein ME, Sutherland D, Mak C, Sidlofsky S, et al. neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. Toronto Breast Cancer Study Group. J Clin Oncol. 1998;16(4):1340–9.
Paterson MC, Dietrich KD, Danyluk J, Paterson AH, Lees AW, Jamil N, et al. Correlation between c-erbB-2 amplification and risk of recurrent disease in node-negative breast cancer. Cancer Res. 1991;51(2):556–67.
Joensuu H, Isola J, Lundin M, Salminen T, Holli K, Kataja V, et al. Amplification of erbB2 and erbB2 expression are superior to estrogen receptor status as risk factors for distant recurrence in pT1N0M0 breast cancer: a nationwide population-based study. Clin Cancer Res. 2003;9(3):923–30.
Colleoni M, Rotmensz N, Peruzzotti G, Maisonneuve P, Viale G, Renne G, et al. Minimal and small size invasive breast cancer with no axillary lymph node involvement: the need for tailored adjuvant therapies. Ann Oncol. 2004;15:1633–9.
Chia S, Norris B, Speers C, Cheang M, Gilks B, Gown AM, et al. Human epidermal growth factor receptor 2 over-expression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol. 2008;26(35):5697–704. Epub 2008 Nov 10.
Amar S, Ann ME, Geiger XJ, Rebecca MB, Winston T, Kyle CE, Beiyun C, Boughey JC, Edith PA. Prognosis and clinical outcome of patients with node negative ≤1 cm breast cancer. Program and abstracts of the 30th annual San Antonio breast cancer symposium, 13–16 Dec 2007, San Antonio, Texas. Abstract 6024.
Tovey SM, Marla S, Brown S Burton P, Canney P, Stallard S, Mallon E, Edwards J, Cooke T. Poor survival outcomes in HER2 positive breast cancer patients with low G, node negative tumours. Implications for trastuzumab. Program and abstracts of the 30th annual San Antonio breast cancer symposium, 13–16 Dec 2008, San Antonio, Texas. Abstract 702.
Ananthakrishnan P, Patrick RJ, Rybicki LA, Tubbs RR, Crowe JP, Budd GT. HER2 amplification does not alter outcome in small (≤1 cm) tumors. Program and abstracts of the 31st annual San Antonio breast cancer symposium, 10–14 Dec 2008, San Antonio, Texas. Abstract 6058.
Gonzalez-Angulo AM, Litton JK, Broglio KR, Meric-Bernstam F, Rakkhit R, Cardoso F, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27(34):5700–6.
Curigliano G, Viale G, Bagnardi V, Fumagalli L, Locatelli M, Rotmensz N, et al. Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol. 2009;27(34):5693–9.
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American society of clinical oncology/college of american pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–45.
Moeder CB, Giltnane JM, Harigopal M, Molinaro A, Robinson A, Gelmon K, et al. Quantitative justification of the change from 10% to 30% for human epidermal growth factor receptor 2 scoring in the American Society of Clinical Oncology/College of American Pathologists guidelines: tumor heterogeneity in breast cancer and its implications for tissue microarray based assessment of outcome. J Clin Oncol. 2007;25(34):5418–25.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98(19):10869–74.
van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999–2009.
Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36.
Untch M, Gelber RD, Jackisch C, Procter M, Baselga J, Bell R, et al. Estimating the magnitude of trastuzumab effects within patient subgroups in the HERA trial. Ann Oncol. 2008;19:1090–6.
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Rolski J, Chan A, Mackey J, Liu M, Pinter T, Valero V, Falkson C et al. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC→T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2neu positive early breast cancer patients: BCIRG 006 study. Abstract 62 presented at the CTRC-AACR annual San Antonio breast cancer symposium, San Antonio, Texas, USA, 9–13 Dec 2009.
Perez EA, Suman VJ, Davidson NE, Gralow J, Kaufman PA, Ingle JN, Dakhil SR, Zujewski JA, Pisansky TM, Jenkins RB. Results of chemotherapy alone, with sequential or concurrent addition of 52 weeks of trastuzumab in the NCCTG N9831 HER2-positive adjuvant breast cancer trial. Abstract 80 presented at the CTRC-AACR annual San Antonio breast cancer symposium, San Antonio, Texas, USA, 9–13 Dec 2009.
Hicks DG, Tubbs RR. Assessment of the HER2 status in breast cancer by fluorescence in situ hybridization: a technical review with interpretive guidelines. Hum Pathol. 2005;36(3):250–61.
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–312.
Rodrigues MJ, Albiges-Sauvin L, Stevens D, Brain E, Delaloge S, Mathieu M, Guillot E, Vincent-Salomon A, Cottu PH. Treatment of node-negative infra-centimetric HER2+ invasive breast carcinomas: a joint AERIO/REMAGUS study. J Clin Oncol 2009; 27:15s, (suppl; abstr 517).
Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28(1):92–8.
Viani GA, Afonso SL, Stefano EJ, De Fendi LI, Soares FV. Adjuvant trastuzumab in the treatment of her-2-positive early breast cancer: a meta-analysis of published randomized trials. BMC Cancer. 2007;7:153.
Acknowledgments
We thank Miss. Daniela Mazza for the grammatical review of the text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petrelli, F., Barni, S. Should adjuvant trastuzumab be offered in very early-stage (pT1a/bN0M0) HER2-neu-positive breast cancer? A current debate. Med Oncol 28, 401–408 (2011). https://doi.org/10.1007/s12032-010-9460-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9460-0