Abstract
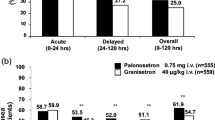
The objective of this study was to compare the efficacy and tolerability of palonosetron and granisetron in a Chinese population receiving highly emetogenic cisplatin-based chemotherapy or moderately emetogenic chemotherapy. Patients were stratified by chemotherapy with cisplatin (yes/no) and then randomly assigned to receive either palonosetron (0.25 mg i.v.) in the first cycle followed by granisetron (3 mg i.v.) in the second cycle or vice versa. The primary efficacy endpoint was the proportion of patients with complete response 0–24 h post-chemotherapy administration. The proportions of patients with complete response 24–120 and 0–120 h following chemotherapy were also compared. Of the 144 patients randomized, 36 (25%) received 60–80 mg/m2 cisplatin; 66 of 72 patients in the palonosetron to granisetron group and 56 of 72 patients in the granisetron to palonosetron group completed treatment with both antiemetics. The efficacy and safety analyses included 128 palonosetron treatments and 138 granisetron treatments. Palonosetron consistently produced numerically higher complete response rates than granisetron in the acute phase (0–24 h, 71.09 vs. 65.22%), the delayed phase (24–120 h, 60.16 vs. 55.80%), and overall (0–120 h, 53.13 vs. 50.00%) though the differences were not significant. Both palonosetron and granisetron were well tolerated. Palonosetron was well tolerated and effective in preventing acute and delayed chemotherapy-induced nausea and vomiting in a Chinese population. When used as monotherapy, 0.25-mg palonosetron was not inferior to 3-mg granisetron for preventing vomiting following highly or moderately emetogenic chemotherapy.



Similar content being viewed by others
References
Grunberg SM, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261–8.
Hesketh PJ, Gralla RJ, Bois AD, Tonato M. Methodology of antiemetic trials: response assessment, evaluation of new agents and definition of chemotherapy emetogenicity. Support Care Cancer. 1998;6:221–7.
Kris MG, et al. American society of clinical oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol. 2006;24:2932–47.
Herrstedt J, Aapro MS, Roila F, Kataja VV. ESMO minimum clinical recommendations for prophylaxis of chemotherapy-induced nausea and vomiting (NV). Ann Oncol. 2005;16(Suppl 1):i77–9.
Eisenberg P, MacKintosh FR, Ritch P, Cornett PA, Macciocchi A. Efficacy, safety and pharmacokinetics of palonosetron in patients receiving highly emetogenic cisplatin-based chemotherapy: a dose-ranging clinical study. Ann Oncol. 2004;15:330–7.
Stoltz R, Cyong JC, Shah A, Parisi S. Pharmacokinetic and safety evaluation of palonosetron, a 5-hydroxytryptamine-3 receptor antagonist, in US and Japanese healthy subjects. J Clin Pharmacol. 2004;44:520–31.
Gralla R, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol. 2003;14:1570–7.
Eisenberg P, et al. Improved prevention of moderately emetogenic chemotherapy induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer. 2003;98:2473–82.
Aapro MS, et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol. 2006;17:1441–9.
Saito M, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a doubleblind, double-dummy, randomised, comparative phase III trial. Lancet Oncol. 2009;10:115–24.
Yu Z, et al. The efficacy and safety of palonosetron compared with granisetron in preventing highly emetogenic chemotherapy-induced vomiting in the Chinese cancer patients: a phase II, multicenter, randomized, double-blind, parallel, comparative clinical trial. Support Care Cancer. 2009;17:99–102.
Cassidy J, et al. Pharmacokinetics and anti-emetic efficacy of BRL43694, a new selective 5HT-3 antagonist. Br J Cancer. 1988;58:651–3.
Hesketh PJ. Defining the emetogenicity of cancer chemotherapy regimens: relevance to clinical practice. Oncologist. 1999;4:191–6.
The Italian Group for Antiemetic Research. Dexamethasone, granisetron, or both for the prevention of nausea and vomiting during chemotherapy for cancer. The Italian Group for Antiemetic Research. N Engl J Med. 1995;332:1–5.
Hesketh PJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, doubleblind, placebo-controlled trial in patients receiving high-dose cisplatin—the aprepitant protocol 052 study group. J Clin Oncol. 2003;21:4112–9.
Navari R, et al. Comparative clinical trial of granisetron and ondansetron in the prophylaxis of cisplatin-induced emesis. The Granisetron study group. J Clin Oncol. 1995;13:1242–8.
Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–94.
Acknowledgments
This trial was funded by Jiuyuan Gene Co., Limited (Hangzhou, China).
Conflicts of interest statement
The authors declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Contributors
Binghua Su is a medical statistical adviser and gave advice on the study design and statistical analyses. Yanyan Su is a medical statistician, mainly responsible for the data analyses. Yahong Chen is the study monitor and coordinated this multicenter study. Ran Xu is the trial member responsible for preservation and distribution of study drugs. All authors identified patients for the trial and critically reviewed the report. This trial is registered with ClinicalTrials.gov number NCT00503386.
Rights and permissions
About this article
Cite this article
Tian, W., Wang, Z., Zhou, J. et al. Randomized, double-blind, crossover study of palonosetron compared with granisetron for the prevention of chemotherapy-induced nausea and vomiting in a Chinese population. Med Oncol 28, 71–78 (2011). https://doi.org/10.1007/s12032-009-9398-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-009-9398-2