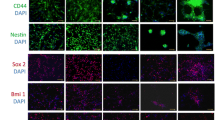
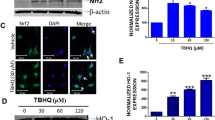
Abstract
Glioblastoma multiforme (GBM) is a prevalent and aggressive primary brain tumor, presenting substantial treatment challenges and high relapse rates. GBM is characterized by alterations in molecular signaling and enzyme expression within malignant cells. This tumor exhibits elevated nitric oxide (NO.) levels. NO. is a crucial signaling molecule involved in the regulation of neuronal functions, synaptic transmission, and cell proliferation. It is primarily synthesized from L-arginine by nitric oxide synthase (NOS) enzymes. The increased levels of NO. in GBM stem from dysregulated activity and expression of clinically relevant NOS isoforms, particularly inducible NOS (iNOS) and neuronal NOS (nNOS). Based on this knowledge, we hypothesize that targeted pharmacological intervention with N6-(1-iminoethyl)-L-lysine (L-NIL), an iNOS inhibitor, and 7-Nitroindazole (7-NI), an nNOS inhibitor, may suggest a promising therapeutic strategy for the treatment of GBM. To test our hypothesis, we utilized the U87-MG cell line as an in vitro model of GBM. Our results showed that treatment with L-NIL and 7-NI led to a reduction in NO. levels, NOS activity, and clonogenic proliferation in U87-MG cells. These findings suggest that NO. and NOS enzymes might be prospective therapeutic targets for GBM.





Similar content being viewed by others
Data Availability
N/A.
References
Abdel-Haq M et al (2023) Effects of extended-release 7-nitroindazole gel formulation treatment on the behavior of shank3 mouse model of autism. Nitric Oxide
Alexander BM, Cloughesy TF (2017) Adult glioblastoma. J Clin Oncol 35(21):2402–2409
Amal H (2022) Sex and the brain: novel ADNP syndrome mice are protected by NAP. Biol Psychiatry 92(1):8–9
Amal H et al (2019) S-nitrosylation of E3 ubiquitin-protein ligase RNF213 alters non-canonical Wnt/Ca+2 signaling in the P301S mouse model of tauopathy. Transl Psychiatry 9(1):44
Amal H et al (2020) Shank3 mutation in a mouse model of autism leads to changes in the S-nitroso-proteome and affects key proteins involved in vesicle release and synaptic function. Mol Psychiatry 25(8):1835–1848
Amal H et al (2020) Low doses of arsenic in a mouse model of human exposure and in neuronal culture lead to S-nitrosylation of synaptic proteins and apoptosis via nitric oxide. Int J Mol Sci 21(11)
Badn W, Siesjö P (2010) The dual role of nitric oxide in glioma. Curr Pharm Des 16(4):428–430
Biserova K et al (2021) Cancer stem cells: significance in origin, pathogenesis and treatment of glioblastoma. Cells 10(3)
Broholm H et al (2003) Nitric oxide synthase expression and enzymatic activity in human brain tumors. Clin Neuropathol 22(6):273–281
Chachlaki K, Prevot V (2020) Nitric oxide signalling in the brain and its control of bodily functions. Br J Pharmacol 177(24):5437–5458
Chen R et al (2017) Glioma subclassifications and their clinical significance. Neurotherapeutics 14(2):284–297
Chen H et al (2023) A nitric-oxide driven chemotactic nanomotor for enhanced immunotherapy of glioblastoma. Nat Commun 14(1):941
Cobbs CS et al (1995) Expression of nitric oxide synthase in human central nervous system tumors. Cancer Res 55(4):727–730
Eyler CE et al (2011) Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell 146(1):53–66
Fahey JM, Korytowski W, Girotti AW (2019) Upstream signaling events leading to elevated production of pro-survival nitric oxide in photodynamically-challenged glioblastoma cells. Free Radic Biol Med 137:37–45
Fahey JM and Girotti AW (2019) Nitric oxide antagonism to anti-glioblastoma photodynamic therapy: mitigation by inhibitors of nitric oxide generation. Cancers (Basel) 11(2)
Gately L et al (2016) Age alone is not a predictor for survival in glioblastoma. J Neurooncol 129(3):479–485
Hamoudi W et al (2021) Regional differences in S-nitrosylation in the cortex, striatum, and hippocampus of juvenile male mice. J Mol Neurosci 71(11):2383–2392
Hamoudi W et al (2022) A cross-talk between nitric oxide and the glutamatergic system in a Shank3 mouse model of autism. Free Radic Biol Med 188:83–91
Hassn Mesrati M et al (2020) Understanding glioblastoma biomarkers: knocking a mountain with a hammer. Cells 9(5)
Jain S, Chalif EJ, Aghi MK (2021) Interactions between anti-angiogenic therapy and immunotherapy in glioblastoma. Front Oncol 11:812916
Jin L et al (2018) CD70, a novel target of CAR T-cell therapy for gliomas. Neuro Oncol 20(1):55–65
Kannan MS, Guiang S, Johnson DE (1998) Nitric oxide: biological role and clinical uses. Indian J Pediatr 65(3):333–345
Kartawy M, Khaliulin I, Amal H (2020) Systems biology reveals reprogramming of the S-nitroso-proteome in the cortical and striatal regions of mice during aging process. Sci Rep 10(1):13913
Kartawy M Khaliulin I and Amal H (2021) Systems biology reveals s-nitrosylation-dependent regulation of mitochondrial functions in mice with shank3 mutation associated with autism spectrum disorder. Brain Sci 11(6)
Kerschbaum HH et al (2000) NADPH-diaphorase activity and nitric oxide synthase activity in the kidney of the clawed frog. Xenopus Laevis Cell Tissue Res 301(3):405–411
Khaliulin I, Kartawy M and Amal H (2020) Sex differences in biological processes and nitrergic signaling in mouse brain. Biomedicines 8(5)
Khaliulin I et al (2021) Preconditioning or postconditioning with 8-Br-cAMP-AM protects the heart against regional ischemia and reperfusion: a role for mitochondrial permeability transition. Cells 10(5)
Kielbik M, Szulc-Kielbik I and Klink M (2019) The potential role of iNOS in ovarian cancer progression and chemoresistance. Int J Mol Sci 20(7)
Kostourou V et al (2011) The role of tumour-derived iNOS in tumour progression and angiogenesis. Br J Cancer 104(1):83–90
Lam-Himlin D et al (2006) Malignant glioma progression and nitric oxide. Neurochem Int 49(8):764–768
Le Rhun E et al (2019) Molecular targeted therapy of glioblastoma. Cancer Treat Rev 80:101896
Lens SM et al (1996) Phenotype and function of human B cells expressing CD70 (CD27 ligand). Eur J Immunol 26(12):2964–2971
Lisi L et al (2017) Expression of iNOS, CD163 and ARG-1 taken as M1 and M2 markers of microglial polarization in human glioblastoma and the surrounding normal parenchyma. Neurosci Lett 645:106–112
Liu X et al (2021) Protein phosphorylation in cancer: role of nitric oxide signaling pathway. Biomolecules 11(7)
López-Collazo E et al (1997) Requirement of nitric oxide and calcium mobilization for the induction of apoptosis in adrenal vascular endothelial cells. FEBS Lett 413(1):124–128
Luo C et al (2021) The prognosis of glioblastoma: a large, multifactorial study. Br J Neurosurg 35(5):555–561
Ma Y et al (2021) Advanced immunotherapy approaches for glioblastoma. Advanced Therapeutics 4(8)
Maccallini C et al (2020) Targeting iNOS As a valuable strategy for the therapy of glioma. ChemMedChem 15(4):339–344
MacMicking J, Xie QW, Nathan C (1997) Nitric oxide and macrophage function. Annu Rev Immunol 15:323–350
Mazurek M, Rola R (2021) The implications of nitric oxide metabolism in the treatment of glial tumors. Neurochem Int 150:105172
McKinnon C et al (2021) Glioblastoma: clinical presentation, diagnosis, and management. BMJ 374:n1560
Mencer S et al (2021) Proteomics of autism and Alzheimer’s mouse models reveal common alterations in mTOR signaling pathway. Transl Psychiatry 11(1):480
Merenzon MA et al (2023) Nitric oxide synthase inhibitors as potential therapeutic agents for gliomas: a systematic review. Nitric Oxide
Omuro A, DeAngelis LM (2013) Glioblastoma and other malignant gliomas: a clinical review. JAMA 310(17):1842–1850
Ostrom QT et al (2014) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol 16(4):iv1–63
Perry A, Wesseling P (2016) Histologic classification of gliomas. Handb Clin Neurol 134:71–95
Pratt D et al (2017) Expression of CD70 (CD27L) is associated with epithelioid and sarcomatous features in IDH-wild-type glioblastoma. J Neuropathol Exp Neurol 76(8):697–708
Puram SV et al (2012) STAT3-iNOS signaling mediates EGFRvIII-induced glial proliferation and transformation. J Neurosci 32(23):7806–7818
Rong L, Li N, Zhang Z (2022) Emerging therapies for glioblastoma: current state and future directions. J Exp Clin Cancer Res 41(1):142
Simmonds MJ, Detterich JA, Connes P (2014) Nitric oxide, vasodilation and the red blood cell. Biorheology 51(2–3):121–134
Steinert JR, Amal H (2023) The contribution of an imbalanced redox signalling to neurological and neurodegenerative conditions. Free Radic Biol Med 194:71–83
Tan AC et al (2020) Management of glioblastoma: state of the art and future directions. CA Cancer J Clin 70(4):299–312
Tanriover N et al (2008) Neuronal nitric oxide synthase expression in glial tumors: correlation with malignancy and tumor proliferation. Neurol Res 30(9):940–944
Tran AN et al (2017) NOS expression and NO function in glioma and implications for patient therapies. Antioxid Redox Signal 26(17):986–999
Tripathi MK, Kartawy M, Amal H (2020) The role of nitric oxide in brain disorders: Autism spectrum disorder and other psychiatric, neurological, and neurodegenerative disorders. Redox Biol 34:101567
Tripathi MK et al (2022) Arsenic alters nitric oxide signaling similar to autism spectrum disorder and Alzheimer’s disease-associated mutations. Transl Psychiatry 12(1):127
Tripathi MK et al (2023) The NO answer for autism spectrum disorder. Adv Sci (weinh) 10(22):e2205783
Wann A et al (2018) Outcomes after second surgery for recurrent glioblastoma: a retrospective case-control study. J Neurooncol 137(2):409–415
Weller M et al (2015) Glioma Nat Rev Dis Primers 1:15017
Wick W et al (2017) Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med 377(20):1954–1963
Wirsching HG, Galanis E, Weller M (2016) Glioblastoma. Handb Clin Neurol 134:381–397
Wu W et al (2021) Glioblastoma multiforme (GBM): an overview of current therapies and mechanisms of resistance. Pharmacol Res 171:105780
Yang H et al (2022) Mechanistic insight into female predominance in Alzheimer’s disease based on aberrant protein S-nitrosylation of C3. Sci Adv 8(50):eade0764
Zhu LJ, Li F and Zhu DY (2023) nNOS and neurological, neuropsychiatric disorders: a 20-year story. Neurosci Bull 1–15
Funding
This work was funded by an Israeli Science Foundation (ISF) grant, an Eagles Autism Foundation grant, a National Institute of Psychobiology in Israel (NIPI) grant, an Israeli Council for Higher Education Maof grant, and a Berettler Centre for Research in Molecular Pharmacology and Therapeutics grant. The authors also thank the Satell Family and the Neubauer Family Foundation.
Author information
Authors and Affiliations
Contributions
DK: conducted experiments, manuscript preparation SKO: conducted experiments MK conducted experiments MKT: conducted experiments, manuscript preparation WH: conducted experiments WB: conducted experiments IG: manuscript preparation HA: project supervision, manuscript preparationAll authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
All animals used were approved by the University Ethical Committee.
Informed Consent
N/A
Competing interests
The research was partially funded by an American Pharma Company under the terms of a Research and License agreement.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kruglyakov, D., Ojha, S.K., Kartawy, M. et al. Nitric Oxide Synthase Inhibition Prevents Cell Proliferation in Glioblastoma. J Mol Neurosci 73, 875–883 (2023). https://doi.org/10.1007/s12031-023-02166-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-023-02166-3