Abstract
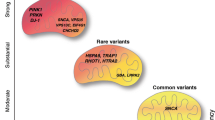
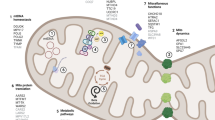
Parkinson’s disease (PD) is speculated with genetic and environmental factors. At molecular level, the mitochondrial impact is stated to be one of the causative reasons for PD. In this study, we investigated the mitochondrial membrane potential (MMP), reactive oxygen species (ROS) and adenosine triphosphate (ATP) levels along with mitochondrial tRNA alterations among three age categories of PD. By determining the genetic and organellar functionality using molecular techniques, the ROS levels were reported to be high with decreased MMP and ATP in the late-onset age group than in other two age categories. Likewise, the tRNA significancy in tRNAThr and tRNAGln was noticed with C4335T and G15927A mutations in late-onset and early-onset PD groups respectively. Therefore, from the findings, ageing has shown a disruption in tRNA metabolism leading to critical functioning of ATP synthesis and MMP, causing oxidative stress in PD patients. These physiological outcomes show that ageing has a keen role in the divergence of mitochondrial function, thereby proving a correlation with ageing and maintenance of mitochondrial homeostasis in PD.




Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Angelova PR, Horrocks MH, Klenerman D et al (2015) Lipid peroxidation is essential for α-synuclein-induced cell death. Journal of neurochemistry 133:582–589
Bingol B, Sheng M (2016) Mechanisms of mitophagy: PINK1, Parkin, USP30 and beyond. Free Radical Biology and Medicine 100:210–222
Cassarino DS, Fall CP, Swerdlow RH et al (1997) Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson’s disease. Biochim Biophys Acta (BBA) Mol Basis Dis 1362:77–89
Cossarizza A, Baccaranicontri M, Kalashnikova G, Franceschi C (1993) A new method for the cytofluorometric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5, 5′, 6, 6′-tetrachloro-1, 1′, 3, 3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1). Biochemical and biophysical research communications 197:40–45
Darzynkiewicz Z, Bedner E, Smolewski P (2001) Flow cytometry in analysis of cell cycle and apoptosis. Elsevier, pp 179–193
Egensperger R, Kösel S, Schnopp NM et al (1997) Association of the mitochondrial tRNA(A4336G) mutation with Alzheimer’s and Parkinson’s diseases. Neuropathol Appl Neurobiol 23:315–321
Gandhi S, Wood-Kaczmar A, Yao Z et al (2009) PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell 33:627–638. https://doi.org/10.1016/j.molcel.2009.02.013
Garone C, Pietra A, Nesci S (2022) From the structural and (Dys) function of ATP synthase to deficiency in age-related diseases. Life (Basel) 12. https://doi.org/10.3390/life12030401
Hällberg BM, Larsson N-G (2014) Making proteins in the powerhouse. Cell Metab 20:226–240. https://doi.org/10.1016/j.cmet.2014.07.001
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11:298–300. https://doi.org/10.1093/geronj/11.3.298
Helley MP, Pinnell J, Sportelli C, Tieu K (2017) Mitochondria: a common target for genetic mutations and environmental toxicants in Parkinson’s disease. Front Genet 8:177
Huang H, Chen J, Lu H et al (2017) Iron-induced generation of mitochondrial ROS depends on AMPK activity. Biometals 30:623–628. https://doi.org/10.1007/s10534-017-0023-0
Hutchin T, Cortopassi G (1995) A mitochondrial DNA clone is associated with increased risk for Alzheimer disease. Proc Natl Acad Sci 92:6892–6895
Je G, Kim Y-S (2017) Mitochondrial ROS-mediated post-transcriptional regulation of α-synuclein through miR-7 and miR-153. Neurosci Lett 661:132–136. https://doi.org/10.1016/j.neulet.2017.09.065
Jiang H, Liu L, Sun DL et al (2016) Interaction between passive smoking and folic acid supplement during pregnancy on autism spectrum disorder behaviors in children aged 3 years. Zhonghua Liu Xing Bing Xue Za Zhi 37:940–944. https://doi.org/10.3760/cma.j.issn.0254-6450.2016.07.007
Junn E, Mouradian MM (2002) Human α-synuclein over-expression increases intracellular reactive oxygen species levels and susceptibility to dopamine. Neurosci Lett 320:146–150
Kroemer G, Zamzami N, Susin SA (1997) Mitochondrial control of apoptosis. Immunol Today 18:44–51
Ludtmann MH, Angelova PR, Horrocks MH et al (2018) α-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat Commun 9:2293
Ma KY, Fokkens MR, Reggiori F, Mari M, Verbeek DS (2021) Parkinson’s disease–associated VPS35 mutant reduces mitochondrial membrane potential and impairs PINK1/Parkin-mediated mitophagy. Transl Neurodegener 10(1):1–7
Magee R, Londin E, Rigoutsos I (2019) TRNA-derived fragments as sex-dependent circulating candidate biomarkers for Parkinson’s disease. Parkinsonism Relat Disord 65:203–209. https://doi.org/10.1016/j.parkreldis.2019.05.035
Mahalaxmi I, Subramaniam MD, Gopalakrishnan AV, Vellingiri B (2021) Dysfunction in mitochondrial electron transport chain complex I, pyruvate dehydrogenase activity, and mutations in ND1 and ND4 gene in autism spectrum disorder subjects from Tamil Nadu Population, India. Mol Neurobiol 58:5303–5311. https://doi.org/10.1007/s12035-021-02492-w
Mayr-Wohlfart U, Paulus C, Henneberg A, Rödel G (1996) Mitochondrial DNA mutations in multiple sclerosis patients with severe optic involvement. Acta Neurol Scand 94:167–171. https://doi.org/10.1111/j.1600-0404.1996.tb07048.x
Mayr-Wohlfart U, Rödel G, Henneberg A (1997) Mitochondrial tRNA (Gln) and tRNA(Thr) gene variants in Parkinson’s disease. Eur J Med Res 2:111–113
Nicholls DG (2004) Mitochondrial membrane potential and aging. Aging Cell 3:35–40. https://doi.org/10.1111/j.1474-9728.2003.00079.x
Paldor I, Madrer N, Vaknine Treidel S et al (2023) Cerebrospinal fluid and blood profiles of transfer RNA fragments show age, sex and Parkinson’s disease-related changes. J Neurochem 164:671–83
Panel M, Ghaleh B, Morin D (2018) Mitochondria and ageing: a role for the mitochondrial transition pore? Ageing Cell 17:e12793
Qadri R, Goyal V, Behari M et al (2021) Alteration of mitochondrial function in oxidative stress in parkinsonian neurodegeneration: a cross-sectional study. Ann Indian Acad Neurol 24:506
Qadri R, Namdeo M, Behari M et al (2018) Alterations in mitochondrial membrane potential in peripheral blood mononuclear cells in Parkinson’s disease: potential for a novel biomarker. Restor Neurol Neurosci 36:719–727. https://doi.org/10.3233/RNN-180852
Ricci J-E, Muñoz-Pinedo C, Fitzgerald P et al (2004) Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell 117:773–786
Richter G, Sonnenschein A, Grünewald T et al (2002) Novel mitochondrial DNA mutations in Parkinson’s disease. J Neural Transm 109:721–729
Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A (1997) JC-1, but not DiOC6 (3) or rhodamine 123, is a reliable fluorescent probe to assess ΔΨ changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett 411:77–82
Shoffner JM, Brown MD, Torroni A et al (1993) Mitochondrial DNA variants observed in Alzheimer disease and Parkinson disease patients. Genomics 17:171–184. https://doi.org/10.1006/geno.1993.1299
Simon DK, Mayeux R, Marder K et al (2000) Mitochondrial DNA mutations in complex I and tRNA genes in Parkinson’s disease. Neurology 54:703–709. https://doi.org/10.1212/wnl.54.3.703
Suski JM, Lebiedzinska M, Bonora M et al (2012) Relation between mitochondrial membrane potential and ROS formation. Mitochondrial bioenergetics: methods and protocols 183–205
Vellingiri B, Suriyanarayanan A, Selvaraj P et al (2022a) Role of heavy metals (copper (Cu), arsenic (As), cadmium (Cd), iron (Fe) and lithium (Li)) induced neurotoxicity. Chemosphere 134625
Vellingiri B, Chandrasekhar M, Sabari SS et al (2022b) Neurotoxicity of pesticides–a link to neurodegeneration. Ecotoxicol Environ Saf 243:113972
Vellingiri B, Suriyanarayanan A, Abraham KS et al (2022c) Influence of heavy metals in Parkinson’s disease: an overview. J Neurolo 1–14
Vellingiri B, Kiruthika B, Mahalaxmi I et al (2023) Role of telomeres and telomerase in Parkinson’s disease - a new theranostics? Adv Biol 2300097
Venkatesan D, Iyer M, Narayanasamy A et al (2022) Genotypic-phenotypic analysis, metabolic profiling and clinical correlations in Parkinson’s disease patients from Tamil Nadu population, India. J Mol Neurosci 1–14
Venkatesan D, Iyer M, Wilson R et al (2021) The association between multiple risk factors, clinical correlations and molecular insights in Parkinson’s disease patients from Tamil Nadu population, India. Neurosci Lett 755:135903
Wallace DC (1994) Mitochondrial DNA sequence variation in human evolution and disease. Proc Natl Acad Sci 91:8739–8746
Wallace DC (2005) A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39:359–407. https://doi.org/10.1146/annurev.genet.39.110304.095751
Yoon KL, Aprille JR, Ernst SG (1991) Mitochondrial tRNA(thr) mutation in fatal infantile respiratory enzyme deficiency. Biochem Biophys Res Commun 176:1112–1115. https://doi.org/10.1016/0006-291x(91)90399-r
Zhou Z, Sun B, Yu D, Bian M (2021) Roles of tRNA metabolism in aging and lifespan. Cell Death Dis 12:548. https://doi.org/10.1038/s41419-021-03838-x
Acknowledgements
The authors would like to thank Indian Council of Medical Research (ICMR) [grant number: 45/2/2018-Hum/BMS], Government of India and Bharathiar University, Coimbatore to carry out this research work. Also, I would like to thank Karpagam Academy of Higher Education (Deemed to be University), Coimbatore 641021, India, for the revision of the manuscript.
Funding
This work was supported by the Indian Council of Medical Research [grant number: 45/2/2018-Hum/BMS], Government of India.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Dhivya Venkatesan, Mahalaxmi Iyer, and Neethu Raj. The first draft of the manuscript was written by Dhivya Venkatesan and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Bharathiar University (BUHEC-0014/2018; dated: 10.08.2018)
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Venkatesan, D., Iyer, M., Raj, N. et al. Assessment of tRNAThr and tRNAGln Variants and Mitochondrial Functionality in Parkinson’s Disease (PD) Patients of Tamil Nadu Population. J Mol Neurosci 73, 912–920 (2023). https://doi.org/10.1007/s12031-023-02154-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-023-02154-7