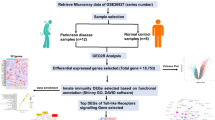
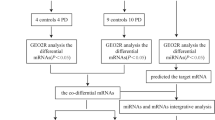
Abstract
Infiltration of CD4 + T cells was found in brain tissue samples from PD patients, suggesting their involvement in developing central nervous system (CNS) disease. The idea of the gut-brain axis further corroborates intestinal T cells’ activation as the central immune response initiation. However, the specific factors and molecular pathways regulating intestinal T-cell activation are unclear. We used the GSE156287 and GSE145814 datasets from the GEO database to analyze and obtain the miRNAs, which are aberrantly expressed in intestinal CD4 + T cells in PD patients and predict their regulatory target mRNAs. Further, combined with the GSE174473 dataset of CD4 + T cells sequencing in PD patients, we finally clarified the aberrant genes expressed in CD4 + T cells from the intestine of PD patients and constructed a miRNA-mRNA regulatory network. The highlight of our findings showed pathways, networks, biological functions, and key molecules potentially involved in the miRNA-mediated functional effects in CD4 + T cell from the intestine of PD patients. The hsa-miR-3180-3p mediated CBX8, etc. were determined as most effective in enhancing T cell survival. PEG10, etc. regulated by hsa-miR-20a-3p targets were possibly involved in T cell differentiation. The JPT2 regulated by hsa-miR-1281 were involved in influencing T cell infiltration. The discovery of this interaction between miRNA and mRNA in CD4 + T cell has important implications for understanding the intestinal initial of PD pathological molecular and anti-inflammation of T cell activation.






Similar content being viewed by others
Data Availability
The data for this paper comes from a public database and is easily accessible to the general public.
References
Arpaia N et al (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504(7480):451–455
Ascoli C et al (2022) Altered transcription factor targeting is associated with differential peripheral blood mononuclear cell proportions in sarcoidosis. Front Immunol 13:848759
Branham-O’Connor M et al (2014) Defective chemokine signal integration in leukocytes lacking activator of G protein signaling 3 (AGS3). J Biol Chem 289(15):10738–10747
Campos-Acuna J, Elgueta D, Pacheco R (2019) T-cell-driven inflammation as a mediator of the gut-brain axis involved in Parkinson’s disease. Front Immunol 10:239
Carvalheiro T et al (2020) Semaphorin4A-plexin D1 axis induces Th2 and Th17 while represses Th1 skewing in an autocrine manner. Int J Mol Sci 21(18)
Chen K et al (2022a) cGAS-STING-mediated IFN-I response in host defense and neuroinflammatory diseases. Curr Neuropharmacol 20(2):362–371
Chen Z et al (2022b) Construction of autophagy-related gene classifier for early diagnosis, prognosis and predicting immune microenvironment features in sepsis by machine learning algorithms. J Inflamm Res 15:6165–6186
Cheng Q et al (2022) The OTUD1-Notch2-ICD axis orchestrates allogeneic T cell-mediated graft-versus-host disease. Blood
Dosil SG et al (2022) MicroRNAs in T cell-immunotherapy. Int J Mol Sci 24(1)
Erttmann SF et al (2022) The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis. Immunity 55(5):847–861 e10
Feng Y et al (2019) Chronic colitis induces meninges traffic of gut-derived T cells, unbalances M1 and M2 microglia/macrophage and increases ischemic brain injury in mice. Brain Res 1707:8–17
Fernandez-Garcia J et al (2022) CD8(+) T cell metabolic rewiring defined by scRNA-seq identifies a critical role of ASNS expression dynamics in T cell differentiation. Cell Rep 41(7):111639
Goetze O, Woitalla D (2008) The role of MPTP in Parkinson’s disease: connecting brain and gut? Exp Neurol 210(2):281–285
Gu L et al (2022) Integrated analysis and identification of critical RNA-binding proteins in bladder cancer. Cancers (Basel) 14(15)
Harms AS et al (2013) MHCII is required for alpha-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci 33(23):9592–9600
Liu Y et al (2011) A novel molecular mechanism for nitrated alpha-synuclein-induced cell death. J Mol Cell Biol 3(4):239–249
Liu F et al (2022) PEG10 amplification at 7q21.3 potentiates large-cell transformation in cutaneous T-cell lymphoma. Blood 139(4):554–571
Lombard-Vadnais F et al (2022) OCA-B does not act as a transcriptional coactivator in T cells. Immunol Cell Biol 100(5):338–351
Luo H et al (2016) EphrinB1 and EphrinB2 regulate T cell chemotaxis and migration in experimental autoimmune encephalomyelitis and multiple sclerosis. Neurobiol Dis 91:292–306
Maehara T et al (2020) Cytotoxic CD4+ T lymphocytes may induce endothelial cell apoptosis in systemic sclerosis. J Clin Invest 130(5):2451–2464
Main BS et al (2016) Type-1 interferons contribute to the neuroinflammatory response and disease progression of the MPTP mouse model of Parkinson’s disease. Glia 64(9):1590–1604
Yang L, Fliegauf M, Grimbacher B (2014) Hyper-IgE syndromes: reviewing PGM3 deficiency. Curr Opin Pediatr 26(6):697–703
Yu T et al (2017) Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 170(3):548–563 e16
Zhao XT et al (2021) Development of a novel 7 immune-related genes prognostic model for oral cancer: a study based on TCGA database. Oral Oncol 112:105088
Funding
This work was supported by the National Natural Science Foundation of China (Grant no: 82101263, to CX Tang), Jiangsu Province Science Foundation for Youths (Grant no: BK20210903, to CX Tang), and Research Foundation for Talented Scholars of Xuzhou Medical University (Grant no: RC20552114, to CX Tang).
National Natural Science Foundation of China,82101263,Jiangsu Province Science Foundation for Youths,BK20210903,Research Foundation for Talented Scholars of Xuzhou Medical University,RC20552114
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Kaiquan Shao and Xinyu Fan. The first draft of the manuscript was written by Kaiquan Shao, Zipeng Wang, and Ruiao Sun, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This is a biographical analysis study and does not require ethical approval.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
The authors affirm that all individual participants provided informed consent for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shao, K., Wang, Z., Fan, X. et al. Comprehensive Analysis of the miRNA-mRNA Pathological Regulatory Network of Intestinal CD4 + T Cells in Parkinson’s Disease. J Mol Neurosci 73, 529–538 (2023). https://doi.org/10.1007/s12031-023-02132-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-023-02132-z