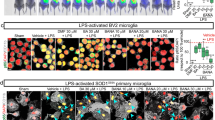
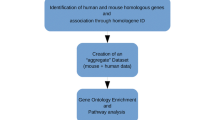
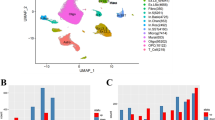
Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal disease of motor neurons that mainly affects the motor cortex, brainstem, and spinal cord. Under disease conditions, microglia could possess two distinct profiles, M1 (toxic) and M2 (protective), with the M2 profile observed at disease onset. SOD1 (superoxide dismutase 1) gene mutations account for up to 20% of familial ALS cases. Comparative gene expression differences in M2-protective (early) stage SOD1G93A microglia and age-matched SOD1G93A motor neurons are poorly understood. We evaluated the differential gene expression profiles in SOD1G93A microglia and SOD1G93A motor neurons utilizing publicly available transcriptomics data and bioinformatics analyses, constructed biomolecular networks around them, and identified gene clusters as potential drug targets. Following a drug repositioning strategy, 5 small compounds (belinostat, auranofin, BRD-K78930611, AZD-8055, and COT-10b) were repositioned as potential ALS therapeutic candidates that mimic the protective state of microglia and reverse the toxic state of motor neurons. We anticipate that this study will provide new insights into the ALS pathophysiology linking the M2 state of microglia and drug repositioning.







Similar content being viewed by others
References
Ahmad L, Zhang SY, Casanova JL, Sancho-Shimizu V (1016) Human TBK1: a gatekeeper of neuroinflammation. Trends in Mol Med 22:511–27
Akhtar RS, Ness JM, Roth KA (2004) Bcl-2 family regulation of neuronal development and neurodegeneration. Biochimica Et Biophysica Acta - Molecular Cell Research 1644:189–203. https://doi.org/10.1016/j.bbamcr.2003.10.013
Amin A, Perera ND, Beart PM, Bradley TJ, Shabanpoor F (2020) Amyotrophic lateral sclerosis and autophagy: dysfunction and therapeutic targeting. Celss 9:2413
Appel S, Zhao W, Beers D, Henkel J (2011) The Microglial-Motoneuron dialogue in ALS. Acta Myologica 4–8
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM et al (2000) Gene ontology: tool for the unification of biology. Nat Genet 25:25–29. https://doi.org/10.1038/75556
Bader GD, Hogue CW (2003) An automated method for finding molecular complexes in large protein interaction networks
Barrett T, Suzek TO, Troup DB, Wilhite SE, Ngau W-C, Ledoux P et al (2005) NCBI GEO: mining millions of expression profiles—database and tools. Nucleic Acids Res 33:D562–D566
Bennett SA, Tanaz R, Cobos SN, Torrente MP (2019) Epigenetics in amyotrophic lateral sclerosis: a role for histone post-translational modifications in neurodegenerative disease. Transl Res 204:19–30. https://doi.org/10.1016/j.trsl.2018.10.002
Bergström P, von Otter M, Nilsson S, Nilsson AC, Nilsson M, Andersen PM et al (2014) Association of NFE2L2 and KEAP1 haplotypes with amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration 15:130–137. https://doi.org/10.3109/21678421.2013.839708
Bolstad BM, Irizarry RA, Astrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. 19
Bono S, Feligioni M, Corbo M (2021) Impaired antioxidant KEAP1-NRF2 system in amyotrophic lateral sclerosis: NRF2 activation as a potential therapeutic strategy. Neurodegeneration 16
Bostock H, Sharief MK, Reid G, Murray NMF (1995) Axonal ion channel dysfunction in amyotrophic lateral sclerosis. 118
Bruneteau G, Simonet T, Bauché S, Mandjee N, Malfatti E, Girard E et al (2013) Muscle histone deacetylase 4 upregulation in amyotrophic lateral sclerosis: potential role in reinnervation ability and disease progression. Brain 136:2359–2368. https://doi.org/10.1093/brain/awt164
Carbon S, Douglass E, Good BM, Unni DR, Harris NL, Mungall CJ et al (2021) The Gene Ontology resource: enriching a gold mine. Nucleic Acids Res 49:D325–D334. https://doi.org/10.1093/nar/gkaa1113
Chin C-H, Chen S-H, Wu H-H, Ho C-W, Ko M-T, Lin C-Y (2014) cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol 8(Suppl 4):S11. https://doi.org/10.1186/1752-0509-8-S4-S11
Ching JK, Weihl CC (2013) Rapamycin-induced autophagy aggravates pathology and weakness in a mouse model of VCP-associated myopathy. Autophagy 9:799–800. https://doi.org/10.4161/auto.23958
Chiu IM, Morimoto ETA, Goodarzi H, Liao JT, O’Keeffe S, Phatnani HP et al (2013) A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep 4:385–401. https://doi.org/10.1016/j.celrep.2013.06.018
Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, Critchlow SE et al (2010) AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Can Res 70:288–298. https://doi.org/10.1158/0008-5472.CAN-09-1751
Coppedè F, Migliore L (2010) DNA repair in premature aging disorders and neurodegeneration. Curr Aging Sci 3:3–19
Craddock TJA, Harvey JM, Nathanson L, Barnes ZM, Klimas NG, Fletcher MA et al (2015) Using gene expression signatures to identify novel treatment strategies in gulf war illness. BMC Med Genom 8. https://doi.org/10.1186/s12920-015-0111-3
Dangoumau A, Marouillat S, Coelho R, Wurmser F, Brulard C, Haouari S et al (2021) Dysregulations of expression of genes of the ubiquitin/SUMO pathways in an in vitro model of amyotrophic lateral sclerosis combining oxidative stress and SOD1 gene mutation. Int J Mol Sci 22:1796
Davis AP, Grondin CJ, Johnson RJ, Sciaky D, McMorran R, Wiegers J et al (2019) The comparative toxicogenomics database: update 2019. Nucleic Acids Res 47:D948–D954
Du L, Zhang Y, Chen Y, Zhu J, Yang Y, Zhang HL (2017) Role of microglia in neurological disorders and their potentials as a therapeutic target. Mol Neurobiol 54:7567–7584. https://doi.org/10.1007/s12035-016-0245-0
Duan Q, Reid SP, Clark NR, Wang Z, Fernandez NF, Rouillard AD et al (2016) L1000CDS2: LINCS L1000 characteristic direction signatures search engine. Npj Systems Biology and Applications 2:16015. https://doi.org/10.1038/npjsba.2016.15
ElBasiouny SM, Schuster JE, Heckman CJ (2010) Persistent inward currents in spinal motoneurons: important for normal function but potentially harmful after spinal cord injury and in amyotrophic lateral sclerosis. Clin Neurophysiol 121:1669–1679. https://doi.org/10.1016/j.clinph.2009.12.041
Eve DJ, Dennis JS, Citron BA (2007) Transcription factor p53 in degenerating spinal cords. Brain Res 1150:174–181
Fabregat A, Sidiropoulos K, Garapati P, Gillespie M, Hausmann K, Haw R et al (2016) The Reactome pathway Knowledgebase. Nucleic Acids Res 44:D481–D487. https://doi.org/10.1093/nar/gkv1351
Fochi S, Bergamo E, Serena M, Mutascio S, Journo C, Mahieux R et al (2019) TRAF3 Is Required for NF-κB pathway activation mediated by HTLV tax proteins. Front Microbiol 10:1302
Gassmann R, Carvalho A, Henzing AJ, Ruchaud S, Hudson DF, Honda R et al (2004) Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. J Cell Biol 166:179–191
Gautier L, Cope L, Bolstad BM, Irizarry RA (2004) Affy - Analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20:307–315. https://doi.org/10.1093/bioinformatics/btg405
Geloso MC, Corvino V, Marchese E, Serrano A, Michetti F, D’Ambrosi N (2017) The dual role of microglia in ALS: Mechanisms and therapeutic approaches. Front Aging Neurosci 9. https://doi.org/10.3389/fnagi.2017.00242
Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S et al (2004) Open access bioconductor: open software development for computational biology and bioinformatics. 5
Granatiero V, Sayles NM, Savino AM, Konrad C, Kharas MG, Kawamata H et al (2021a) Modulation of the IGF1R-MTOR pathway attenuates motor neuron toxicity of human ALS SOD1G93A astrocytes. Autophagy 17:4029–4042
Grover MP, Ballouz S, Mohanasundaram KA, George RA, Goscinski A, Crowley TM et al (2015) Novel therapeutics for coronary artery disease from genome-wide association study data. BMC Med Genom 8. https://doi.org/10.1186/1755-8794-8-S2-S1
Halon-Golabek M, Borkowska A, Kaczor JJ, Ziolkowski W, Flis DJ, Knap N et al (2018) hmSOD1 gene mutation-induced disturbance in iron metabolism is mediated by impairment of Akt signalling pathway. J Cachexia Sarcopenia Muscle 9:557–569. https://doi.org/10.1002/jcsm.12283
Henkel JS, Beers DR, Siklós L, Appel SH (2006) The chemokine MCP-1 and the dendritic and myeloid cells it attracts are increased in the mSOD1 mouse model of ALS. Mol Cell Neurosci 31:427–437. https://doi.org/10.1016/j.mcn.2005.10.016
Henriques A, Kastner S, Chatzikonstantinou E, Pitzer C, Plaas C, Kirsch F et al (2015) Gene expression changes in spinal motoneurons of the SOD1G93A transgenic model for ALS after treatment with G-CSF. Front Cell Neurosci 8:1–12. https://doi.org/10.3389/fncel.2014.00464
Hetz C, Thielen P, Fisher J, Pasinelli P, Brown RH, Korsmeyer S et al (2007) The proapoptotic BCL-2 family member BIM mediates motoneuron loss in a model of amyotrophic lateral sclerosis. Cell Death Differ 14:1386–1389. https://doi.org/10.1038/sj.cdd.4402166
Honda R, Korner R, Nigg EA (2003) Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell 14:3325–3341
Iczkiewicz J, Rose S, Jenner P (2005) Increased osteopontin expression following intranigral lipopolysaccharide injection in the rat. Eur J Neurosci 21:1911–1920. https://doi.org/10.1111/j.1460-9568.2005.04009.x
Imamura K, Izumi Y, Watanabe A, Tsukita K, Woltjen K, Yamamoto T et al (2017) The Src/c-Abl pathway is a potential therapeutic target in amyotrophic lateral sclerosis. Sci Trans Med 1–10
Inoue H, Tsukita K, Iwasato T, Suzuki Y, Tomioka M, Tateno M et al (2003) The crucial role of caspase-9 in the disease progression of a transgenic ALS mouse model. EMBO J 22:6665–6674
Ishigaki S, Liang Y, Yamamoto M, Niwa J-I, Ando Y, Yoshihara T et al (2002) X-Linked inhibitor of apoptosis protein is involved in mutant SOD1-mediated neuronal degeneration. J Neurochem 82:576–584
Jagaraj CJ, Parakh S, Atkin JD (2021) Emerging evidence highlighting the importance of redox dysregulation in the pathogenesis of amyotrophic lateral sclerosis (ALS). Front Cellular Neurosci 14
Jiang YM, Yamamoto M, Kobayashi Y, Yoshihara T, Liang Y, Terao S et al (2005) Gene expression profile of spinal motor neurons in sporadic amyotrophic lateral sclerosis. Ann Neurol 57:236–251. https://doi.org/10.1002/ana.20379
Källstig E, McCabe BD, Schneider BL (2021) The links between als and nf-κb. Int J Mol Sci 22. https://doi.org/10.3390/ijms22083875
Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M et al (2008) KEGG for linking genomes to life and the environment. Nucleic Acids Res 36:D480–D484. https://doi.org/10.1093/nar/gkm882
Kaur SJ, McKeown SR, Rashid S (2016) Mutant SOD1 mediated pathogenesis of Amyotrophic Lateral Sclerosis. Gene 577:109–118. https://doi.org/10.1016/j.gene.2015.11.049
Kikuchi H, Yamada T, Furuya H, Doh-Ura K, Ohyagi Y, Iwaki T et al (2003) Involvement of cathepsin B in the motor neuron degeneration of amyotrophic lateral sclerosis. Acta Neuropathol 105:462–468. https://doi.org/10.1007/s00401-002-0667-9
Killoy KM, Harlan BA, Pehar M, Vargas MR (2022) NR1D1 downregulation in astrocytes induces a phenotype that is detrimental to cocultured motor neurons. FASEB J 36:e22262
Kim S, Chen J, Cheng T, Gindulyte A, He J, He S et al (2019) PubChem 2019 update: improved access to chemical data. Nucleic Acids Res 47:D1102–D1109. https://doi.org/10.1093/nar/gky1033
Kirby J, Ning K, Ferraiuolo L, Heath PR, Ismail A, Kuo S-W et al (2011) Phosphatase and tensin homologue/protein kinase B pathway linked to motor neuron survival in human superoxide dismutase 1-related amyotrophic lateral sclerosis. Brain 134:506–517
Klingl YE, Pakravan D, Van Den BL (2021) Opportunities for histone deacetylase inhibition in amyotrophic lateral sclerosis. Br J Pharmacol 178:1353–1372. https://doi.org/10.1111/bph.15217
Klingl YE, Pakravan D, Van Den Bosch L (2020) Opportunities for histone deacetylase inhibition in amyotrophic lateral sclerosis. British J Pharmacol 178:1353–72. https://doi.org/10.1111/bph.v178.6/issuetoc
Knott C, Stern G, Wilkin GP (2000) Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci 16:724–739. https://doi.org/10.1006/mcne.2000.0914
Konopka A, Atkin JD (2022) DNA damage, defective DNA repair, and neurodegeneration in amyotrophic lateral sclerosis. Front Aging Neurosci 14:786420. https://doi.org/10.3389/fnagi.2022.786420
Kubat Öktem E, Mruk K, Chang J, Akin A, Kobertz WR, Brown RH (2016) Mutant SOD1 protein increases Nav1.3 channel excitability. J Biol Phys 42:351–70. https://doi.org/10.1007/s10867-016-9411-x
Laird AS, van Hoecke A, de Muynck L, Timmers M, van den Bosch L, van Damme P et al (2010) Progranulin is neurotrophic in vivo and protects against a mutant TDP-43 induced axonopathy. PLoS ONE 5. https://doi.org/10.1371/journal.pone.0013368
Lee HZ, Kwitkowski VE, del Valle PL, Ricci MS, Saber H, Habtemariam BA et al (2015) FDA approval: belinostat for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma. Clin Cancer Res 21:2666–2670. https://doi.org/10.1158/1078-0432.CCR-14-3119
Lee JD, Kamaruzaman NA, Fung JNT, Taylor SM, Turner BJ, Atkin JD et al (2013) Dysregulation of the complement cascade in the hSOD1G93A transgenic mouse model of amyotrophic lateral sclerosis. J Neuroinflamm 10. https://doi.org/10.1186/1742-2094-10-119
Liao B, Zhao W, Beers DR, Henkel JS, Appel SH (2012) Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp Neurol 237:147–152. https://doi.org/10.1016/j.expneurol.2012.06.011
Liu J, Lillo C, Andreas Jonsson P, vande Velde C, Ward CM, Miller TM et al (2004) Toxicity of Familial ALS-Linked SOD1 Mutants from Selective Recruitment to Spinal Mitochondria mitochondria as the basis for their selective toxicity in ALS. 43
Lorenzl S, Narr S, Angele B, Krell HW, Gregorio J, Kiaei M et al (2006) The matrix metalloproteinases inhibitor Ro 26–2853 extends survival in transgenic ALS mice. Exp Neurol 200:166–171. https://doi.org/10.1016/j.expneurol.2006.01.026
Madeira JM, Bajwa E, Stuart MJ, Hashioka S, Klegeris A (2014) Gold drug auranofin could reduce neuroinflammation by inhibiting microglia cytotoxic secretions and primed respiratory burst. J Neuroimmunol 276:71–79. https://doi.org/10.1016/j.jneuroim.2014.08.615
Madeira JM, Renschler CJ, Mueller B, Hashioka S, Gibson DL, Klegeris A (2013) Novel protective properties of auranofin: Inhibition of human astrocyte cytotoxic secretions and direct neuroprotection. Life Sci 92:1072–1080. https://doi.org/10.1016/j.lfs.2013.04.005
Maetzler W, Berg D, Schalamberidze N, Melms A, Schott K, Mueller JC et al (2007) Osteopontin is elevated in Parkinson’s disease and its absence leads to reduced neurodegeneration in the MPTP model. Neurobiol Dis 25:473–482. https://doi.org/10.1016/j.nbd.2006.10.020
Maiese K (2015) FoxO proteins in the nervous system. Anal Cellular Pathol 2015. https://doi.org/10.1155/2015/569392
Manzano R, Toivonen JM, Calvo AC, Oliván S, Zaragoza P, Rodellar C et al (2013) Altered in vitro proliferation of mouse SOD1-G93A skeletal muscle satellite cells. Neurodegener Dis 11:153–164
Meller R, Stevens SL, Minami M, Cameron JA, King S, Rosenzweig H et al (2005) Neuroprotection by osteopontin in stroke. J Cereb Blood Flow Metab 25:217–225. https://doi.org/10.1038/sj.jcbfm.9600022
Mitchell J, Morris A, de Belleroche J (2009) Thioredoxin reductase 1 haplotypes modify familial amyotrophic lateral sclerosis onset. Free Radical Biol Med 46:202–211. https://doi.org/10.1016/j.freeradbiomed.2008.09.041
Mojsilovic-Petrovic J, Nedelsky N, Boccitto M, Mano I, Georgiades SN, Zhou W et al (2009) FOXO3a is broadly neuroprotective in vitro and in vivo against insults implicated in motor neuron diseases. J Neurosci 29:8236–8247. https://doi.org/10.1523/JNEUROSCI.1805-09.2009
Moon GJ, Shin M, Kim SR (2020) Upregulation of neuronal Rheb(S16H) for hippocampal protection in the adult brain. Int J Mol Sci 21. https://doi.org/10.3390/ijms21062023
Mouton AJ, Rivera Gonzalez OJ, Kaminski AR, Moore ET, Lindsey ML (2018) Matrix metalloproteinase-12 as an endogenous resolution promoting factor following myocardial infarction. Pharmacol Res 137:252–258. https://doi.org/10.1016/j.phrs.2018.10.026
Natarajan R, Singal V, Benes R, Gao J, Chan H, Chen H et al (2014) STAT3 modulation to enhance motor neuron differentiation in human neural stem cells. PLoS ONE 9. https://doi.org/10.1371/journal.pone.0100405
Noristani HN, Sabourin JC, Gerber YN, Teigell M, Sommacal A, Vivanco MDM et al (2015) Brca1 is expressed in human microglia and is dysregulated in human and animal model of ALS. Mol Neurodegener 10:34. https://doi.org/10.1186/s13024-015-0023-x
Oberstadt M, Stieler J, Simpong DL, Römuß U, Urban N, Schaefer M et al (2018) TDP-43 self-interaction is modulated by redox-active compounds Auranofin, Chelerythrine and Riluzole. Sci Reps 8. https://doi.org/10.1038/s41598-018-20565-0
Öktem EK, Yazar M, Gulfidan G, Arga KY (2019) Cancer Drug Repositioning by Comparison of Gene Expression in Humans and Axolotl (Ambystoma mexicanum) During Wound Healing. OMICS 23:389–405. https://doi.org/10.1089/omi.2019.0093
Onodera T, Momose I, Kawada M (2019) Potential Anticancer Activity of Auranofin. 67
Oprea TI, Overington JP (2015) Computational and practical aspects of drug repositioning. Assay Drug Dev Technol 13:299–306. https://doi.org/10.1089/adt.2015.29011.tiodrrr
Oughtred R, Stark C, Breitkreutz B-J, Rust J, Boucher L, Chang C et al (2019) The BioGRID interaction database: 2019 update. Nucleic Acids Res 47:D529–D541
Pasinelli P, Houseweart MK, Brown RH, Cleveland DW (2000) Caspase-1 and-3 are sequentially activated in motor neuron death in Cu, Zn superoxide dismutase-mediated familial amyotrophic lateral sclerosis. PNAS 97:13901–13906
Piscopo P, Crestini A, Adduci A, Ferrante A, Massari M, Popoli P et al (2011) Altered oxidative stress profile in the cortex of mice fed an enriched branched-chain amino acids diet: Possible link with amyotrophic lateral sclerosis? J Neurosci Res 89:1276–1283. https://doi.org/10.1002/jnr.22655
Prudencio M, Hart PJ, Borchelt DR, Andersen PM (2009) Variation in aggregation propensities among ALS-associated variants of SOD1: Correlation to human disease. Hum Mol Genet 18:3217–3226. https://doi.org/10.1093/hmg/ddp260
Quintero-Villegas A, Valdés-Ferrer SI (2022) Central nervous system effects of 5-HT7 receptors: a potential target for neurodegenerative diseases. Mol Med 28
Roder C, Thomson MJ (2015) Auranofin: Repurposing an Old Drug for a Golden New Age. Drugs in R and D 15:13–20. https://doi.org/10.1007/s40268-015-0083-y
Rollo EE, Denhart DT (1996) Differential effects of osteopontin on the cytotoxic activity of macrophages from young and old mice. Immunology 88:642–647
Romano R, Bucci C (2020) Role of EGFR in the Nervous System. Cells 9:1887
Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A (1993) Mutations in CuZn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362:59–62
Rosen EY, Wexler EM, Versano R, Coppola G, Gao F, Winden KD et al (2011) Functional Genomic Analyses Identify Pathways Dysregulated by Progranulin Deficiency. Implicating Wnt Signaling Neuron 71:1030–1042. https://doi.org/10.1016/j.neuron.2011.07.021
Rouaux C, Panteleeva I, René F, de Aguilar JLG, Echaniz-Laguna A, Dupuis L et al (2007) Sodium valproate exerts neuroprotective effects in vivo through CREB-binding protein-dependent mechanisms but does not improve survival in an amyotrophic lateral sclerosis mouse model. J Neurosci 27:5535–5545. https://doi.org/10.1523/JNEUROSCI.1139-07.2007
Ryan CL, Baranowski DC, Chitramuthu BP, Malik S, Li Z, Cao M et al (2009) Progranulin is expressed within motor neurons and promotes neuronal cell survival. BMC Neurosci 10:130. https://doi.org/10.1186/1471-2202-10-130
Ryu H, Smith K, Camelo SI, Carreras I, Lee J, Iglesias AH et al (2005) Sodium phenylbutyrate prolongs survival and regulates expression of anti-apoptotic genes in transgenic amyotrophic lateral sclerosis mice. J Neurochem 93:1087–1098. https://doi.org/10.1111/j.1471-4159.2005.03077.x
Sahaan TG, Zhang KE (2021) Mitogen-Activated Protein Kinase Pathway in Amyotrophic Lateral Sclerosis. Biomedicines 9:969
Sakaguchi T, Irie T, Kawabata R, Yoshida A, Maruyama H, Kawakami H (2011) Optineurin with amyotrophic lateral sclerosis-related mutations abrogates inhibition of interferon regulatory factor-3 activation. Neurosci Lett 505:279–281. https://doi.org/10.1016/j.neulet.2011.10.040
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D et al (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504. https://doi.org/10.1101/gr.1239303
Shibata N, Kakita A, Takahashi H, Ihara Y, Nobukuni K, Fujimura H et al (2009) Activation of signal transducer and activator of transcription-3 in the spinal cord of sporadic amyotrophic lateral sclerosis patients. Neurodegener Dis 6:118–126. https://doi.org/10.1159/000213762
Shlevkov E, Basu H, Bray M-A, Sun Z, Wei W, Apaydin K et al (2019) A High-Content Screen Identifies TPP1 and Aurora B as Regulators of Axonal Mitochondrial Transport. Cell Rep 28:3224–3237.e5. https://doi.org/10.1016/j.celrep.2019.08.035
Shukla S, Tekwani BL (2020) Histone Deacetylases Inhibitors in Neurodegenerative Diseases, Neuroprotection and Neuronal Differentiation. Front Pharmacol 11. https://doi.org/10.3389/fphar.2020.00537
Smedley D, Haider S, Durinck S, Pandini L, Provero P, Allen J et al (2015) The BioMart community portal: An innovative alternative to large, centralized data repositories. Nucleic Acids Res 43:W589–W598. https://doi.org/10.1093/nar/gkv350
Smyth GK, Ritchie M, Thorne N, Wettenhall J (2005) LIMMA: linear models for microarray data. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Stat Biol Heal
Sreedharan J, Brown RH (2013) Amyotrophic lateral sclerosis: Problems and prospects. Ann Neurol 74:309–316. https://doi.org/10.1002/ana.24012
Sullivan PM, Zhou X, Hu F (2017) Autophagy-Lysosome Dysfunction in Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degeneration. Lysosomes - Associated Diseases and Methods to Study Their Function, InTech. https://doi.org/10.5772/intechopen.69371
Szklarczyk D, Santos A, von Mering C, Jensen LJ, Bork P, Kuhn M (2016) STITCH 5: Augmenting protein-chemical interaction networks with tissue and affinity data. Nucleic Acids Res 44:D380–D384. https://doi.org/10.1093/nar/gkv1277
Tortarolo M, Vallarola A, Lidonnici D, Battaglia E, Gensano F, Spaltro G et al (2015) Lack of TNF-alpha receptor type 2 protects motor neurons in a cellular model of amyotrophic lateral sclerosis and in mutant SOD1 mice but does not affect disease progression. J Neurochem 135:109–124
Trieu VN, Liu R, Liu XP, Uckun FM (2000) A specific inhibitor of Janus kinase-3 increases survival in a transgenic mouse model of amyotrophic lateral sclerosis. Biochem Biophys Res Commun 267:22–25. https://doi.org/10.1006/bbrc.1999.1905
Trostchansky A, Mastrogiovanni M, Miquel E, Rodríguez-Bottero S, Martínez-Palma L, Cassina P et al (2018) Profile of Arachidonic Acid-Derived Inflammatory Markers and Its Modulation by Nitro-Oleic Acid in an Inherited Model of Amyotrophic Lateral Sclerosis. Front Mol Neurosci 11
Turanli B, Grøtli M, Boren J, Nielsen J, Uhlen M, Arga KY et al (2018) Drug repositioning for effective prostate cancer treatment. Front Physiol 9. https://doi.org/10.3389/fphys.2018.00500
Turanli B, Karagoz K, Bidkhori G, Sinha R, Gatza ML, Uhlen M et al (2019) Multi-omic data interpretation to repurpose subtype specific drug candidates for breast cancer. Front Gen 10. https://doi.org/10.3389/fgene.2019.00420
Turanli B, Zhang C, Kim W, Benfeitas R, Uhlen M, Arga KY et al (2019) Discovery of therapeutic agents for prostate cancer using genome-scale metabolic modeling and drug repositioning. EBioMedicine 42:386–396. https://doi.org/10.1016/j.ebiom.2019.03.009
Van Kan HJM, Van Den Berg LH, Groeneveld GJ, van der Straaten R, van Vught PWJ, Lie‐A‐Huen L et al (2008) Pharmacokinetics of riluzole: evidence for glucuronidation as a major metabolic pathway not associated with UGT1A1 genotype. Biopharm Drug Dispos 29:139–144
Virit O, Selek S, Bulut M, Savas HA, Celik H, Erel O et al (2008) High ceruloplasmin levels are associated with obsessive compulsive disorder: A case control study. Behav Brain Funct4. https://doi.org/10.1186/1744-9081-4-52
Vogrinc D, Kunej T (2017) Drug repositioning: computational approaches and research examples classified according to the evidence level. Discoveries 5:e75. https://doi.org/10.15190/d.2017.5
von Grabowiecki Y, Abreu P, Blanchard O, Palamiuc L, Benosman S, Mériaux S et al (2016) Transcriptional activator TAp63 is upregulated in muscular atrophy during ALS and induces the pro-atrophic ubiquitin ligase Trim63. Elife 5:e10528. https://doi.org/10.7554/eLife.10528
Wang Y, Tang X, Yu B, Gu Y, Yuan Y, Yao D et al (2012) Gene Network Revealed Involvements of Birc2, Birc3 and Tnfrsf1a in Anti-Apoptosis of Injured Peripheral Nerves. PLoS ONE 7. https://doi.org/10.1371/journal.pone.0043436
Watanabe S, Ageta-Ishihara N, Nagatsu S, Takao K, Komine O, Endo F et al (2014) SIRT1 overexpression ameliorates a mouse model of SOD1-linked amyotrophic lateral sclerosis via HSF1/HSP70i chaperone system. Mol Brain 7. https://doi.org/10.1186/s13041-014-0062-1
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR et al (2018) DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res 46:D1074–82. https://doi.org/10.1093/nar/gkx1037
Wolf FG, dos Santos LOE, Philippi PC (2006) Plasma copper, iron, ceruloplasmin and ferroxidase activity in schizophrenia. Schizophr Res 86:167–171. https://doi.org/10.1016/j.schres.2006.05.027
Xie M, Liu YU, Zhao S, Zhang L, Bosco DB, Pang Y-P et al (2022) TREM2 interacts with TDP-43 and mediates microglial neuroprotection against TDP-43-related neurodegeneration. Nat Neurosci 25:26–38. https://doi.org/10.1038/s41593-021-00975-6
Xu R, Wang QQ (2016) A genomics-based systems approach towards drug repositioning for rheumatoid arthritis. BMC Genomics 17. https://doi.org/10.1186/s12864-016-2910-0
Xu X, Zhang J, Li S, Al-Nusaif M, Zhou Q, Chen S et al (2022) Bone Marrow Stromal Cell Antigen 2: Is a Potential Neuroinflammation Biomarker of SOD1G93A Mouse Model of Amyotrophic Lateral Sclerosis in Pre-symptomatic Stage. Front Neurosci 15. https://doi.org/10.3389/fnins.2021.788730
Yoo YE, Ko CP (2011) Treatment with trichostatin A initiated after disease onset delays disease progression and increases survival in a mouse model of amyotrophic lateral sclerosis. Exp Neurol 231:147–159. https://doi.org/10.1016/j.expneurol.2011.06.003
Zhang M, Luo H, Xi Z, Rogaeva E (2015) Drug repositioning for diabetes based on “omics” data mining. PLoS ONE 10. https://doi.org/10.1371/journal.pone.0126082
Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O et al (2019) Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun 10:1523. https://doi.org/10.1038/s41467-019-09234-6
Author information
Authors and Affiliations
Contributions
EKO conceived the original idea, conceptualized the study, performed analysis, prepared figures and tables, and wrote the manuscript. BA performed analysis, prepared figures and tables, and wrote the manuscript. MY performed analysis and prepared figures and tables. KYA conceptualized the study, supervised the study and improved the final version of the manuscript. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable; the study did not have any participants and relevant information.
Consent to Participate
Not applicable; the study did not have any participants and relevant information.
Consent for Publication
All authors consent for publication in Journal of Molecular Neuroscience.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kubat Oktem, E., Aydin, B., Yazar, M. et al. Integrative Analysis of Motor Neuron and Microglial Transcriptomes from SOD1G93A Mice Models Uncover Potential Drug Treatments for ALS. J Mol Neurosci 72, 2360–2376 (2022). https://doi.org/10.1007/s12031-022-02071-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-022-02071-1