Abstract
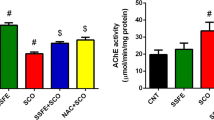
This study evaluates some of the neuromodulatory mechanisms of the memory loss preventive effect of alpha-lipoic acid (ALA) in a scopolamine (Sco)-induced rat model of Alzheimer’s disease (AD) type dementia. Our results confirmed that Sco administration induces significant memory impairment, worsens exploratory behaviour and habituation, increases acetylcholinesterase (AChE) activity, and induces pathological monoamine content changes in the prefrontal cortex and hippocampus. ALA administration largely prevented Sco-induced memory impairment. It also improved exploratory behaviour and preserved habituation, and it decreased AChE activity, reversing it to control group levels, and corrected aberrant monoamine levels in the prefrontal cortex and hippocampus. According to the data available, this is the first time that ALA-induced changes in AChE and monoamine levels in the prefrontal cortex and hippocampus (brain structures related to learning and memory) have been demonstrated in a Sco-induced rat model of AD type dementia.




Similar content being viewed by others
Availability of Data and Materials
The material used during the present study is available from the corresponding author on reasonable request.
Data Availability Statement
The authors confirm that all data generated or analysed during this study are included in this published article.
References
Ahmed H (2012) Modulatory effects of vitamin E, acetyl-l-carnitine and α-lipoic acid on new potential biomarkers for Alzheimer's disease in rat model. Exp Toxicol Pathol 64:549–556. https://doi.org/10.1016/j.etp.2010.11.012
Arendt T (2012) Cell cycle activation and aneuploid neurons in Alzheimer's disease. Mol Neurobiol 46:125–135. https://doi.org/10.1007/s12035-012-8262-0
Ballard C, Greig N, Guillozet-Bongaarts A, Enz A, Darvesh S (2005) Cholinesterases: Roles in the brain during health and disease. Curr Alzheimer Res 2:307–318. https://doi.org/10.2174/1567205054367838
Bartus R, Dean R, Beer B, Lippa A (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217:408–414. https://doi.org/10.1126/science.7046051
Bilska A, Dubiel M, Sokołowska-Jez˙ewicz M, Lorenc-Koci E, Włodek L (2007) Alpha-lipoic acid differently affects the reserpine-induced oxidative stress in the striatum and prefrontal cortex of rat brain. Neuroscience 146:1758–1771. https://doi.org/10.1016/j.neuroscience.2007.04.002
Boissier JR, Simon P, Lwoff JM (1964) L’utilisation d’une réaction particulière de la Souris (méthode de la planche à trous) pour l’étude des médicaments psychotropes. Thérapie 19:571–589
Bozhokina E, Khaitlina S, Gamaley I (2015) Dihydrolipoic but not alpha-lipoic acid affects susceptibility of eukaryotic cells to bacterial invasion. Biochem Biophys Res Commun 460:697–702. https://doi.org/10.1016/j.bbrc.2015.03.092
Braak H, Braak E (1996) Development of Alzheimer-related neurofibrillary changes in the neocortex inversely recapitulates cortical myelogenesis. Acta Neuropathol 92:197–201. https://doi.org/10.1007/s004010050508
Burns J, Galvin J, Roe C, Morris J, McKeel D (2005) The pathology of the substantia nigra in Alzheimer disease with extrapyramidal signs. Neurology 64:1397–1403. https://doi.org/10.1212/01.wnl.0000158423.05224.7f
Chudasama Y, Robbins T (2004) Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacology 29:1628–1636. https://doi.org/10.1038/sj.npp.1300490
D’Amelio M, Rossini P (2012) Brain excitability and connectivity of neuronal assemblies in Alzheimer's disease: From animal models to human findings. Prog Neurobiol 99:42–60. https://doi.org/10.1016/j.pneurobio.2012.07.001
Dinicola S, Proietti S, Cucina A, Bizzarri M, Fuso A (2017) Alpha-lipoic acid downregulates IL-1β and IL-6 by DNA hypermethylation in SK-N-BE neuroblastoma cells. Antioxidants 6:74. https://doi.org/10.3390/antiox6040074
Dragomanova S, Tancheva L, Georgieva M, Georgieva A, Dishovsky C, Stoeva S et al (2016) Preventive effect of myrtenal and lipoic acid in combination on progression of Alzheimer’s disease. Eur Neuropsychopharmacol 26:S636. https://doi.org/10.1016/s0924-977x(16)31732-1
Ebert K (1998) Scopolamine model of dementia: electroencephalogram findings and cognitive performance. Eur J Clin Investig 28:944–949. https://doi.org/10.1046/j.1365-2362.1998.00393.x
Ellman G, Courtney K, Andres V, Featherstone R (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
El-Sherbiny D, Khalifa A, Attia A, Eldenshary E (2003). Hypericum perforatum extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolamine. Pharmacol Biochem Behav 76:525–533. https://doi.org/10.1016/j.pbb.2003.09.014
Fava A, Pirritano D, Plastino M, Cristiano D, Puccio G, Colica C et al (2013) The effect of lipoic acid therapy on cognitive functioning in patients with Alzheimer's disease. J Neurodegener Dis 2013:1–7. https://doi.org/10.1155/2013/454253
Ferreira P, Militão G, Freitas R (2009) Lipoic acid effects on lipid peroxidation level, superoxide dismutase activity and monoamines concentration in rat hippocampus. Neurosci Lett 464:131–134. https://doi.org/10.1016/j.neulet.2009.08.051
Fiedler S, Yadav V, Kerns A, Tsang C, Markwardt S, Kim E et al (2018) Lipoic acid stimulates cAMP production in healthy control and secondary progressive MS subjects. Mol Neurobiol 55:6037–6049. https://doi.org/10.1007/s12035-017-0813-y
Flood J, Cherkin A (1986) Scopolamine effects on memory retention in mice: A model of dementia?. Behav Neural Biol 45:169–184. https://doi.org/10.1016/s0163-1047(86)90750-8
Francis PT, Palmer AM, Snape M, Wilcock GK (1999) The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry 66(2):137–147. https://doi.org/10.1136/jnnp.66.2.137
Fu B, Zhang J, Zhang X, Zhang C, Li Y, Zhang Y et al (2014) Alpha-lipoic acid upregulates SIRT1-dependent PGC-1α expression and protects mouse brain against focal ischemia. Neuroscience 281:251–257. https://doi.org/10.1016/j.neuroscience.2014.09.058
González-Reyes R, Nava-Mesa M, Vargas-Sánchez K, Ariza-Salamanca D, Mora-Muñoz L (2017) Involvement of astrocytes in Alzheimer’s disease from a neuroinflammatory and oxidative stress perspective. Front Mol Neurosci 10. https://doi.org/10.3389/fnmol.2017.00427
Goverdhan P, Sravanthi A, Mamatha T (2012) Neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress. Int J Alzheimers Dis 2012:1–8. https://doi.org/10.1155/2012/974013
Hasselmo ME (2006) The role of acetylcholine in learning and memory. Curr Opin Neurobiol 16(6):710–715. https://doi.org/10.1016/j.conb.2006.09.002
Heneka M, Obanion M (2007) Inflammatory processes in Alzheimer's disease. J Neuroimmunol 184:69–91. https://doi.org/10.1016/j.jneuroim.2006.11.017
Huang W, Zhang X, Chen W (2016) Role of oxidative stress in Alzheimer's disease. Biomed Rep 4:519–522. https://doi.org/10.3892/br.2016.630
Jacobowitz D, Richardson J (1978) Method for the rapid determination of norepinephrine, dopamine, and serotonin in the same brain region. Pharmacol Biochem Behav 8:515–519. https://doi.org/10.1016/0091-3057(78)90380-5
James B, Leurgans S, Hebert L, Scherr P, Yaffe K, Bennett D (2014a) Contribution of Alzheimer disease to mortality in the United States. Neurology 82:1045–1050. https://doi.org/10.1212/wnl.0000000000000240
James BD, Leurgans SE, Hebert LE, Scherr PA, Yaffe K, Bennett DA (2014b) Contribution of Alzheimer disease to mortality in the United States. Neurology 82(12):1045–1050. https://doi.org/10.1212/wnl.0000000000000240
Jarvik M, Kopp R (1967) An improved one-trial passive avoidance learning situation. Psychol Rep 21:221–224. https://doi.org/10.2466/pr0.1967.21.1.221
Jesudason E, Masilamoni J, Kirubagaran R, Davis G, Jayakumar R (2005) The protective role of dl-α-lipoic acid in biogenic amines catabolism triggered by Aβ amyloid vaccination in mice. Brain Res Bull 65:361–367. https://doi.org/10.1016/j.brainresbull.2005.01.010
Lee B, Sur B, Shim I, Lee H, Hahm D (2012) Phellodendron amurense and its major alkaloid compound, berberine ameliorates scopolamine-induced neuronal impairment and memory dysfunction in rats. Korean J Physiol Pharmacol 16:79. https://doi.org/10.4196/kjpp.2012.16.2.79
Lowry O, Rosebrough N, Farr A, Randall R (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275. https://doi.org/10.1016/s0021-9258(19)52451-6
Mancuso M, Coppede F, Migliore L, Siciliano G, Murri L (2006) Mitochondrial dysfunction, oxidative stress and neurodegeneration. J Alzheimers Dis 10:59–73. https://doi.org/10.3233/jad-2006-10110
Meng J, Ni J, Wu Z, Jiang M, Zhu A, Qing H et al (2018) The critical role of IL-10 in the antineuroinflammatory and antioxidative effects of rheum tanguticum on activated microglia. Oxid Med Cell Longev 2018:1–12. https://doi.org/10.1155/2018/1083596
Miquel J (2002) Can antioxidant diet supplementation protect against age-related mitochondrial damage?. Ann N Y Acad Sci 959:508–516. https://doi.org/10.1111/j.1749-6632.2002.tb02120.x
Molz P, Schröder N (2017) Potential therapeutic effects of lipoic acid on memory deficits related to aging and neurodegeneration. Front Pharmacol 8. https://doi.org/10.3389/fphar.2017.00849
Nordberg A, Nyberg P, Adolfsson R, Winblad B (1987) Cholinergic topography in Alzheimer brains: a comparison with changes in the monoaminergic profile. J Neural Transm 69:19–32. https://doi.org/10.1007/bf01244094
Nordberg A, Winblad B (1986) Reduced number of [3H]nicotine and [3H]acetylcholine binding sites in the frontal cortex of Alzheimer brains. Neurosci Lett 72:115–120. https://doi.org/10.1016/0304-3940(86)90629-4
Ray P, Huang B, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24:981–990. https://doi.org/10.1016/j.cellsig.2012.01.008
Romanski LM, LeDoux JE (1993) Information cascade from primary auditory cortex to the amygdala: Corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb Cortex 3:515–532
Roy D, Arons A, Mitchell T, Pignatelli M, Ryan T, Tonegawa S (2016) Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 531:508–512. https://doi.org/10.1038/nature17172
Sara S (2009) The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 10:211–223. https://doi.org/10.1038/nrn2573
Sara S (2017) Sleep to Remember. J Neurosci 37:457–463. https://doi.org/10.1523/jneurosci.0297-16.2017
Scheff S, Price D, Schmitt F, Mufson E (2006) Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol Aging 27:1372–1384. https://doi.org/10.1016/j.neurobiolaging.2005.09.012
Schicknick H, Henschke J, Budinger E, Ohl F, Gundelfinger E, Tischmeyer W (2019) β‐adrenergic modulation of discrimination learning and memory in the auditory cortex. Eur J Neurosci 50:3141–3163. https://doi.org/10.1111/ejn.14480
Serrano-Pozo A, Frosch M, Masliah E, Hyman B (2011) Neuropathological alterations in alzheimer disease. Cold Spring Harb Perspect Med 1:a006189–a006189. https://doi.org/10.1101/cshperspect.a006189
Shivakumar S, Ilango K, Agrawal A, Dubey GP (2014) Effect of hippophae rhamnoides on cognitive enhancement via neurochemical modulation in scopolamine induced Sprague Dawely rats. Int J Pharm Sci Res 5. https://doi.org/10.13040/ijpsr.0975-8232.5(10).4153-58
Spinks A, Wasiak J (2011) Scopolamine (hyoscine) for preventing and treating motion sickness. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.cd002851.pub4
Tsvetanova E, Alexandrova A, Georgieva A, Tancheva L, Lazarova M, Dolashka P, Velkova L, Dolashki A, Atanasov V, Kalfin R (2020) Effect of mucus extract of Helix aspersa on scopolamine-induced cognitive impairment and oxidative stress in rat’s brain. Bulg Chem Commun 52:107–111
Tzvetanova ER, Georgieva AP, Alexandrova AV, Tancheva LP, Lazarova MI, Dragomanova ST, Alova LG, Stefanova MO, Kalfin RE (2018) Antioxidant mechanisms in neuroprotective action of lipoic acid on learning and memory of rats with experimental dementia. Bulg Chem Commun 50:52–57
Upadhyay P, Shukla R, Tiwari K, Dubey G, Mishra S (2020) Neuroprotective effect of Reinwardtia indica against scopolamine induced memory-impairment in rat by attenuating oxidative stress. Metab Brain Dis 35:709–725. https://doi.org/10.1007/s11011-019-00479-0
Zaki HF, Abd-El-Fattah MA, Attia AS (2014) Naringenin protects against scopolamine-induced dementia in rats. Bull Fac Pharm Cairo Univ 52(1):15–25. https://doi.org/10.1016/j.bfopcu.2013.11.001
Acknowledgements
This work is supported by the Bulgarian Ministry of Education and Science under the National Program for Research “Young Scientists and Postdoctoral Students”.
Funding
This work is supported by the Bulgarian Ministry of Education and Science under the National Program for Research “Young Scientists and Postdoctoral Students”.
Author information
Authors and Affiliations
Contributions
Conception and design [Lyubka Tancheva], [Maria Lazarova], and [Rumen Nikolov]. Material preparation, data collection and analysis were performed by [L. Tancheva], [M. Lazarova], [Hristian Staykov], [Yozljam Hassanova], [Miroslava Stefanova]. The first draft of the manuscript was written by [M. Lazarova] and all authors comment on previous version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The experiments have been performed strictly according to the national regulations and European Communities Council Directive (86/609/EEC) also Principles of laboratory animal care (NIH publication No. 85-23) concerning the protection of animals used for scientific and experimental purposes. All efforts and study design was made with purpose to minimize number of the animals and their suffering.
Consent for Publication
The authors declare their consent for publication.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Staykov, H., Lazarova, M., Hassanova, Y. et al. Neuromodulatory Mechanisms of a Memory Loss-Preventive Effect of Alpha-Lipoic Acid in an Experimental Rat Model of Dementia. J Mol Neurosci 72, 1018–1025 (2022). https://doi.org/10.1007/s12031-022-01979-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-022-01979-y