Abstract
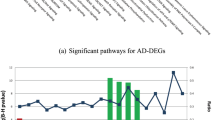
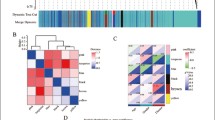
Neuroinflammation-induced neurodegeneration and immune cell infiltration are two features of Alzheimer disease (AD). This study aimed to identify potential peripheral biomarkers that interact with cerebrospinal fluid (CSF) and infiltrating immune cells in AD. Blood and CSF data were downloaded from the Alzheimer’s disease Neuroimaging Initiative database. We identified differentially expressed genes (DEGs) in AD and assessed infiltrating immune cells using the Immune Cell Abundance Identifier (ImmuCellAI) algorithm. Blood-brain barrier (BBB) and immune-related genes were identified from medical databases, and common genes were used to construct a protein-protein interaction network (PPI). Potential biomarkers reflecting the clinical features of AD were screened using Pearson correlations and logistic regression analysis. We identified 210 DEGs in the AD group. ImmuCellAI indicated that blood samples from patients with AD had a higher abundance of exhausted T (Tex; 0.196 vs. 0.132) and induced regulatory T (iTreg; 0.180 vs. 0.137) cells than controls. Thirty-two genes overlapped between the BBB and immune-related genes, and 27 genes in the PPI network were associated with eight pathways, including the cytokine-cytokine receptor interaction pathway (hsa04060) and the chemokine signaling pathway (hsa04062). Pearson correlations showed that five genes were associated with the CSF biomarkers, Aβ, total, and phosphorylated tau. Logistics analysis showed that the B cell-associated genes, CXCL12 and TNFRSF13C, were independent risk factors for AD diagnosis. Peripheral CXCL12 and TNFRSF13C genes that correlated with immune cell infiltration in AD might serve as easily accessible biomarkers for the early diagnosis of AD.




Similar content being viewed by others
Data Availability
The original microarray datasets of Alzheimer disease (including GSE4226, GSE4229, and GSE18309) are available from the National Center of Biotechnology Information Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/). Peripheral blood, CSF, and plasma data of patients with Alzheimer disease and controls were downloaded from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (http://adni.loni.usc.edu/).
References
Balietti M, Giuli C, Conti F (2018) Peripheral blood brain-derived neurotrophic factor as a biomarker of Alzheimer’s disease: are there methodological biases? Mol Neurobiol 55:6661–6672. https://doi.org/10.1007/s12035-017-0866-y
Balsa-Canto E, Henriques D, Gábor A, Banga JR (2016) AMIGO2, a toolbox for dynamic modeling, optimization and control in systems biology. Bioinformatics 32:3357–3359
Calsolaro V, Edison P (2016) Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement 12:719–732
Dong X, Nao J, Shi J, Zheng D (2019) Predictive value of routine peripheral blood biomarkers in Alzheimer’s disease. Front Aging Neurosci 11. https://doi.org/10.3389/fnagi.2019.003322019.00332
Fisher Y, Nemirovsky A, Baron R, Monsonego A (2010) T cells specifically targeted to amyloid plaques enhance plaque clearance in a mouse model of Alzheimer’s disease. PLoS One 5:e10830
Garg B et al (2018) NFκB in pancreatic stellate cells reduces infiltration of tumors by cytotoxic T cells and killing of cancer cells, via up-regulation of CXCL12. Gastroenterology 155:880–891.e888. https://doi.org/10.1053/j.gastro.2018.05.0512018.05.051
González H, Pacheco R (2014) T-cell-mediated regulation of neuroinflammation involved in neurodegenerative diseases. J Neuroinflammation 11:201
Heneka MT et al (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14:388–405. https://doi.org/10.1016/S1474-4422(15)70016-5
Higham JP, Malik BR, Buhl E, Dawson JM, Ogier AS, Lunnon K, Hodge JJL (2019) Alzheimer’s disease associated genes ankyrin and tau cause shortened lifespan and memory loss in drosophila. Front Cell Neurosci 13:260–260. https://doi.org/10.3389/fncel.2019.00260
Ho GJ, Drego R, Hakimian E, Masliah E (2005) Mechanisms of cell signaling and inflammation in Alzheimer’s disease. Curr Drug Targets: Inflammation Allergy 4:247–256
Huang DW et al (2007) DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res 35:W169–W175
Janssen B et al (2016) Imaging of neuroinflammation in Alzheimer’s disease, multiple sclerosis and stroke: Recent developments in positron emission tomography. Biochim Biophys Acta (BBA) - Mol Basis Dis 1862:425–441. https://doi.org/10.1016/j.bbadis.2015.11.011
Klebanoff CA et al (2005) Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci 102:9571–9576
Liu HL et al (2015) Clinical significance of preoperative CD8+ central memory T cells for operable pancreatic adenocarcinoma. Dig Surg 32 (6):433-438
Marsh SE et al (2016) The adaptive immune system restrains Alzheimer’s disease pathogenesis by modulating microglial function. Proc Natl Acad Sci USA 12:P463–P464
McDade E et al (2018) Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease. Neurology 91:e1295–e1306
Medeiros R, LaFerla FM (2013) Astrocytes: conductors of the Alzheimer disease neuroinflammatory symphony. Exp Neurol 239:133–138
Mei J, Xu R, Xia D, Yang X, Wang H, Liu C (2020) Profiles and clinical significance of immune cell infiltration in pancreatic adenocarcinoma. bioRxiv. https://doi.org/10.1101/2020.1103.1130.017327
Miao YR, Zhang Q, Lei Q, Luo M, Xie GY, Wang H, Guo AY (2019) ImmuCellAI: a unique method for comprehensive T-cell subsets abundance prediction and its application in cancer immunotherapy bioRxiv:872184
Mietelska-Porowska A, Wojda U (2017) T lymphocytes and inflammatory mediators in the interplay between brain and blood in Alzheimer’s disease: potential pools of new biomarkers. J Immunol Res 2017:4626540. https://doi.org/10.1155/2017/4626540
Mihalcik SA, Huddleston PM, Wu X, Jelinek DF (2010) The structure of the TNFRSF13C promoter enables differential expression of BAFF-R during B cell ontogeny and terminal differentiation. J Immunol 185:1045–1054
Ntellas P et al (2020) TNFRSF13C/BAFFR P21R and H159Y polymorphisms in multiple sclerosis. Mult Scler Relat Disord 37:101410–101422. https://doi.org/10.1016/j.msard.102019.101422
Ortiz G et al (2017) Oxidative stress: Love and hate history in central nervous system. Adv Protein Chem Struct Biol 108:1-31. https://doi.org/10.1016/bs.apcsb.2017.01.003
Pepper M, Jenkins MK (2011) Origins of CD4(+) effector and central memory T cells. Nat Immunol 12:467–471. https://doi.org/10.1038/ni.2038
Prinz M, Priller J (2017) The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci 20:136–144. https://doi.org/10.1038/nn.4475
Quiroz YT et al (2018) Association between amyloid and tau accumulation in young adults with autosomal dominant Alzheimer disease. JAMA Neurol 75:548–556
Regen F, Hellmann-Regen J, Costantini E, Reale M (2017) Neuroinflammation and Alzheimer's disease: Implications for microglial activation. Curr Alzheimer Res 14:1140-1148. https://doi.org/10.2174/1567205014666170203141717
Rezai-Zadeh K, Gate D, Town T (2009) CNS infiltration of peripheral immune cells: D-Day for neurodegenerative disease? J Neuroimmune Pharmacol 4:462–475
Rosenberg GA (2012) Neurological diseases in relation to the blood-brain barrier. J Cereb Blood Flow Metab 32:1139–1151
Sallusto F, Geginat J, Lanzavecchia A (2004) Central memory and effector memory T cell subsets: function generation, and maintenance. Annu Rev Immunol 22:745–763. https://doi.org/10.1146/annurev.immunol.22.012703.104702
Sawikr Y, Yarla NS, Peluso I, Kamal MA, Aliev G, Bishayee A (2017) Neuroinflammation in Alzheimer's disease: the preventive and therapeutic potential of polyphenolic nutraceuticals. Adv Protein Chem Struct Biol 108:33-57. https://doi.org/10.1016/bs.apcsb.2017.02.001
Schaerli P, Moser B (2005) Chemokines: control of primary and memory T-cell traffic. Immunol Res 31:57–74
Shi B, Qi J (2020) The pattern and prognostic relevance of immune activity scores and tumor-infiltrating immune cells in metastatic clear cell renal cell carcinoma: evidence from multiple datasets. Int Immunopharmacol 85:106651
Singhal G, Jaehne EJ, Corrigan F, Toben C, Baune BT (2014) Inflammasomes in neuroinflammation and changes in brain function: a focused review. Front Neurosci 8:315
Song YJ et al (2020) Immune landscape of the tumor microenvironment identifies prognostic gene signature CD4/CD68/CSF1R in Osteosarcoma. Front Oncol 10:1198. https://doi.org/10.3389/fonc.2020.01198
Sun S et al (2020) Development and validation of an immune-related prognostic signature in lung adenocarcinoma. Cancer Med 9:5960–5975
Swarbrick S, Wragg N, Ghosh S, Stolzing A (2019) Systematic review of miRNA as biomarkers in Alzheimer’s disease. Mol Neurobiol 56:6156–6167
Turazzi N et al (2018) Engineered T cells towards TNFRSF13C (BAFFR): a novel strategy to efficiently target B-cell acute lymphoblastic leukaemia. Br J Haematol 182:939–943. https://doi.org/10.1111/bjh.14899
Tussiwand R, Rauch M, Flück LA, Rolink AG (2012) BAFF-R expression correlates with positive selection of immature B cells. Eur J Immunol 42:206–216
Wang X, Wang L (2020) Screening and identification of potential peripheral blood biomarkers for Alzheimer’s disease based on bioinformatics analysis. Med Sci Monit 26:e924263. https://doi.org/10.12659/MSM.924263
Yang YM, Shang DS, Zhao WD, Fang WG, Chen YH (2013) Microglial TNF-α-dependent elevation of MHC class I expression on brain endothelium induced by amyloid-beta promotes T cell transendothelial migration. Neurochem Res 38:2295–2304
Yuen SC, Zhu H, Leung SW (2020) A Systematic Bioinformatics Workflow With Meta-Analytics Identified Potential Pathogenic Factors of Alzheimer’s Disease. Front Neurosci 14:209
Zamanian Azodi M, Rezaei-Tavirani M, Rezaei Tavirani M (2020) Investigating the effects of ibuprofen on the gene expression profile in Hippocampus of mice model of Alzheimer’s disease through bioinformatics analysis. Iranian Journal of Pharmaceutical Research 19:352–359
Zou L et al (2004) Bone marrow is a reservoir for CD4+ CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Can Res 64:8451–8455
Funding
This work was supported by Natural Science Foundation of Shanghai (No.18ZR1417200, obtained by Wei Kong) and National Natural Science Foundation of China (No.61803257; obtained by Shuaiqun Wang).
Author information
Authors and Affiliations
Contributions
Conception and design of the research: Qianqian Wu and Wei Kong. Acquisition, analysis, and interpretation of data: Qianqian Wu, Shuaiqun Wang, and Wei Kong. Statistical analysis: Qianqian Wu. Drafting the manuscript: Qianqian Wu. Manuscript revision for important intellectual content: Wei Kong. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This article does not contain any studies with human participants or animals performed by any of the authors; therefore, the ethical approval and consent to participate are not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, Q., Kong, W. & Wang, S. Peripheral Blood Biomarkers CXCL12 and TNFRSF13C Associate with Cerebrospinal Fluid Biomarkers and Infiltrating Immune Cells in Alzheimer Disease. J Mol Neurosci 71, 1485–1494 (2021). https://doi.org/10.1007/s12031-021-01809-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-021-01809-7