Abstract
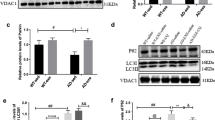
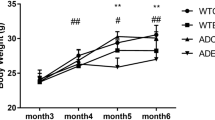
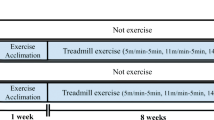
Autophagy/mitophagy, a cellular catabolic process necessary for sustaining normal cellular function, has emerged as a potential therapeutic strategy against numerous obstinate diseases. In this regard, endurance exercise (EXE)-induced autophagy/mitophagy (EIAM) has been considered as a potential health-enriching factor in various tissues including the brain; however, underlying mechanisms of EIAM in the brain has not been fully defined yet. This study investigated the molecular signaling nexus of EIAM pathways in the cortex of the brain. C57BL/6 young male mice were randomly assigned to a control group (CON, n = 12) and an endurance exercise group (EXE, n = 12). Our data demonstrated that exercise-induced autophagy coincided with an enhanced anabolic state (p-AKT, p-mTOR, and p-p70S6K); furthermore, mitophagy concurred with enhanced mitochondrial turnover: increases in both fission (DRP1, BNIP3, and PINK1) and fusion (OPA1 and MFN2) proteins. In addition, neither oxidative stress nor sirtuins (SIRT) 1 and 3 were associated with EIAM; instead, the activation of AMPK as well as a JNK-BCL2 axis was linked to EIAM promotion. Collectively, our results demonstrated that EXE-induced anabolic enrichment did not hinder autophagy/mitophagy and that the concurrent augmentation of mitochondrial fusion and fusion process contributed to sustaining mitophagy in the cortex of the brain. Our findings suggest that the EXE-induced concomitant potentiation of the catabolic and anabolic state is a unique molecular mechanism that simultaneously contributes to recycling and rebuilding the cellular structure, leading to upholding healthy cellular environment. Thus, the current study provides a novel autophagy/mitophagy mechanism, from which groundbreaking pharmacological strategies of autophagy can be developed.





Similar content being viewed by others
References
Akbar M, Essa MM, Daradkeh G, Abdelmegeed MA, Choi Y, Mahmood L, Song BJ (2016) Mitochondrial dysfunction and cell death in neurodegenerative diseases through nitroxidative stress. Brain Res 1637:34–55
Almeida MF, Silva CM, Chaves RS, Lima NCR, Almeida RS, Melo KP, Demasi M, Fernandes T, Oliveira EM, Netto LES, Cardoso SM, Ferrari MFR (2018) Effects of mild running on substantia nigra during early neurodegeneration. J Sports Sci 36:1363–1370
Barazzuol L, Giamogante F, Brini M, Cali T (2020) PINK1/Parkin mediated mitophagy, Ca(2+) signalling, and ER-mitochondria contacts in Parkinson’s disease. Int J Mol Sci 21
Chen H, Chan DC (2009) Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet 18:R169–R176
Chen X, Pan Z, Fang Z, Lin W, Wu S, Yang F, Li Y, Fu H, Gao H, Li S (2018) Omega-3 polyunsaturated fatty acid attenuates traumatic brain injury-induced neuronal apoptosis by inducing autophagy through the upregulation of SIRT1-mediated deacetylation of Beclin-1. J Neuroinflammation 15:310
Chen Y, Meng J, Xu Q, Long T, Bi F, Chang C, Liu W (2019) Rapamycin improves the neuroprotection effect of inhibition of NLRP3 inflammasome activation after TBI. Brain Res 1710:163–172
Ding H, Jiang N, Liu H, Liu X, Liu D, Zhao F, Wen L, Liu S, Ji LL, Zhang Y (2010) Response of mitochondrial fusion and fission protein gene expression to exercise in rat skeletal muscle. Biochim Biophys Acta 1800:250–256
Fan XY, Tian C, Wang H, Xu Y, Ren K, Zhang BY, Gao C, Shi Q, Meng G, Zhang LB, Zhao YJ, Shao QX, Dong XP (2015) Activation of the AMPK-ULK1 pathway plays an important role in autophagy during prion infection. Sci Rep 5:14728
Filomeni G, De Zio D, Cecconi F (2015) Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ 22:377–388
Gao LP, Xiao K, Wu YZ, Chen DD, Yang XH, Shi Q, Dong XP (2020) Enhanced mitophagy activity in prion-infected cultured cells and prion-infected experimental mice via a Pink1/Parkin-dependent mitophagy pathway. ACS Chem Neurosci 11:814–829
Glick D, Barth S, Macleod KF (2010) Autophagy: cellular and molecular mechanisms. J Pathol 221:3–12
He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B (2012a) Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 481:511–515
He C, Sumpter R Jr, Levine B (2012b) Exercise induces autophagy in peripheral tissues and in the brain. Autophagy 8:1548–1551
Hiebel C, Kromm T, Stark M, Behl C (2014) Cannabinoid receptor 1 modulates the autophagic flux independent of mTOR- and BECLIN1-complex. J Neurochem 131:484–497
Inoki K, Kim J, Guan KL (2012) AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol 52:381–400
Jamart C, Naslain D, Gilson H, Francaux M (2013) Higher activation of autophagy in skeletal muscle of mice during endurance exercise in the fasted state. Am J Phys Endocrinol Metab 305:E964–E974
Jang Y, Kwon I, Song W, Cosio-Lima LM, Lee Y (2018) Endurance exercise mediates neuroprotection against MPTP-mediated Parkinson’s disease via enhanced neurogenesis, antioxidant capacity, and autophagy. Neuroscience 379:292–301
Jewell JL, Guan KL (2013) Nutrient signaling to mTOR and cell growth. Trends Biochem Sci 38:233–242
Jonasson LS, Nyberg L, Kramer AF, Lundquist A, Riklund K, Boraxbekk CJ (2016) Aerobic exercise intervention, cognitive performance, and brain structure: results from the physical influences on brain in aging (PHIBRA) study. Front Aging Neurosci 8:336
Ju JS, Jeon SI, Park JY, Lee JY, Lee SC, Cho KJ, Jeong JM (2016) Autophagy plays a role in skeletal muscle mitochondrial biogenesis in an endurance exercise-trained condition. J Physiol Sci: JPS 66:417–430
Juhasz G, Erdi B, Sass M, Neufeld TP (2007) Atg7-dependent autophagy promotes neuronal health, stress tolerance, and longevity but is dispensable for metamorphosis in drosophila. Genes Dev 21:3061–3066
Kim JS, Nitta T, Mohuczy D, O'Malley KA, Moldawer LL, Dunn WA, Jr., Behrns KE (2008) Impaired autophagy: a mechanism of mitochondrial dysfunction in anoxic rat hepatocytes. Hepatology (Baltimore, Md) 47:1725-1736
Kim J, Kundu M, Viollet B, Guan KL (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13:132–141
Koo JH, Cho JY (2017) Treadmill exercise attenuates alpha-synuclein levels by promoting mitochondrial function and autophagy possibly via SIRT1 in the chronic MPTP/P-induced mouse model of Parkinson’s disease. Neurotox Res 32:473–486
Kou X, Li J, Liu X, Chang J, Zhao Q, Jia S, Fan J, Chen N (2017) Swimming attenuates d-galactose-induced brain aging via suppressing miR-34a-mediated autophagy impairment and abnormal mitochondrial dynamics. J Appl Physiol (1985) 122:1462–1469
Kuma A, Komatsu M, Mizushima N (2017) Autophagy-monitoring and autophagy-deficient mice. Autophagy 13:1619–1628
Lee Y, Kang EB, Kwon I, Cosio-Lima L, Cavnar P, Javan GT (2016) Cardiac kinetophagy coincides with activation of anabolic signaling. Med Sci Sports Exerc 48:219–226
Lee Y, Kwon I, Jang Y, Song W, Cosio-Lima LM, Roltsch MH (2018) Correction to: potential signaling pathways of acute endurance exercise-induced cardiac autophagy and mitophagy and its possible role in cardioprotection. J Physiol Sci: JPS 68:205
Lezi E, Swerdlow RH (2012) Mitochondria in neurodegeneration. Adv Exp Med Biol 942:269–286
Li B, Liang F, Ding X, Yan Q, Zhao Y, Zhang X, Bai Y, Huang T, Xu B (2019) Interval and continuous exercise overcome memory deficits related to beta-amyloid accumulation through modulating mitochondrial dynamics. Behav Brain Research 112171
Lloyd BA, Hake HS, Ishiwata T, Farmer CE, Loetz EC, Fleshner M, Bland ST, Greenwood BN (2017) Exercise increases mTOR signaling in brain regions involved in cognition and emotional behavior. Behav Brain Res 323:56–67
Luo L, Dai JR, Guo SS, Lu AM, Gao XF, Gu YR, Zhang XF, Xu HD, Wang Y, Zhu Z, Wood LJ, Qin ZH (2017) Lysosomal proteolysis is associated with exercise-induced improvement of mitochondrial quality control in aged hippocampus. J Gerontol A Biol Sci Med Sci 72:1342–1351
Mandal A, Drerup CM (2019) Axonal transport and mitochondrial function in neurons. Front Cell Neurosci 13:373
Marques-Aleixo I, Santos-Alves E, Balca MM, Rizo-Roca D, Moreira PI, Oliveira PJ, Magalhaes J, Ascensao A (2015) Physical exercise improves brain cortex and cerebellum mitochondrial bioenergetics and alters apoptotic, dynamic and auto(mito)phagy markers. Neuroscience 301:480–495
McMillan EM, Pare MF, Baechler BL, Graham DA, Rush JW, Quadrilatero J (2015) Autophagic signaling and proteolytic enzyme activity in cardiac and skeletal muscle of spontaneously hypertensive rats following chronic aerobic exercise. PLoS One 10:e0119382
Ni B, Shen H, Wang W, Lu H, Jiang L (2019) TGF-beta1 reduces the oxidative stress-induced autophagy and apoptosis in rat annulus fibrosus cells through the ERK signaling pathway. J Orthop Surg Res 14:241
Noda NN, Inagaki F (2015) Mechanisms of autophagy. Annu Rev Biophys 44:101–122
Osiewacz HD, Bernhardt D (2013) Mitochondrial quality control: impact on aging and life span - a mini-review. Gerontology 59:413–420
O'Sullivan TE, Johnson LR, Kang HH, Sun JC (2015) BNIP3- and BNIP3L-mediated mitophagy promotes the generation of natural killer cell memory. Immunity 43:331–342
Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL (2010) Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588:4795–4810
Qiao Z, Wang X, Xiang L, Zhang C (2016) Dysfunction of autophagy: a possible mechanism involved in the pathogenesis of vitiligo by breaking the redox balance of melanocytes. Oxidative Med Cell Longev 2016:3401570
Rocchi A, He C (2017) Regulation of exercise-induced autophagy in skeletal muscle. Curr Pathobiol Rep 5:177–186
Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, Kim H, Neufeld TP, Dillin A, Guan KL (2013) ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol 15:741–750
Ryskalin L, Lazzeri G, Flaibani M, Biagioni F, Gambardella S, Frati A, Fornai F (2017) mTOR-dependent cell proliferation in the brain. Biomed Res Int 2017:7082696
Salminen A, Kaarniranta K (2012) AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev 11:230–241
Sarkar S (2013) Regulation of autophagy by mTOR-dependent and mTOR-independent pathways: autophagy dysfunction in neurodegenerative diseases and therapeutic application of autophagy enhancers. Biochem Soc Trans 41:1103–1130
Shang L, Wang X (2011) AMPK and mTOR coordinate the regulation of Ulk1 and mammalian autophagy initiation. Autophagy 7:924–926
Takei N, Nawa H (2014) mTOR signaling and its roles in normal and abnormal brain development. Front Mol Neurosci 7:28
Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ 3rd, Lotze MT (2010) Endogenous HMGB1 regulates autophagy. J Cell Biol 190:881–892
Thellung S, Corsaro A, Nizzari M, Barbieri F, Florio T (2019) Autophagy activator drugs: a new opportunity in neuroprotection from misfolded protein toxicity. Int J Mol Sci 20
Tseng AH, Shieh SS, Wang DL (2013) SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic Biol Med 63:222–234
Villa E, Proics E, Rubio-Patino C, Obba S, Zunino B, Bossowski JP, Rozier RM, Chiche J, Mondragon L, Riley JS, Marchetti S, Verhoeyen E, Tait SWG, Ricci JE (2017) Parkin-independent mitophagy controls chemotherapeutic response in cancer cells. Cell Rep 20:2846–2859
Watson K, Baar K (2014) mTOR and the health benefits of exercise. Semin Cell Dev Biol 36:130–139
Wei Y, Pattingre S, Sinha S, Bassik M, Levine B (2008) JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell 30:678–688
Xu P, Das M, Reilly J, Davis RJ (2011) JNK regulates FoxO-dependent autophagy in neurons. Genes Dev 25:310–322
Zhang L, Chao FL, Luo YM, Xiao Q, Jiang L, Zhou CN, Zhang Y, Lv FL, He Q, Ma J, Tang Y (2017) Exercise prevents cognitive function decline and demyelination in the white matter of APP/PS1 transgenic AD mice. Curr Alzheimer Res 14:645–655
Zhou J, Tan SH, Nicolas V, Bauvy C, Yang ND, Zhang J, Xue Y, Codogno P, Shen HM (2013) Activation of lysosomal function in the course of autophagy via mTORC1 suppression and autophagosome-lysosome fusion. Cell Res 23:508–523
Funding
This project was supported by a grant from the University of West Florida through the Office of Research and Sponsored Programs (R0036 and R0062).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kwon, I., Jang, Y. & Lee, Y. Endurance Exercise-Induced Autophagy/Mitophagy Coincides with a Reinforced Anabolic State and Increased Mitochondrial Turnover in the Cortex of Young Male Mouse Brain. J Mol Neurosci 71, 42–54 (2021). https://doi.org/10.1007/s12031-020-01624-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-020-01624-6