Abstract
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by the degeneration of motor neurons. Between 12 and 20% of inherited cases and approximately 1–2% of all cases are caused by mutations in the gene encoding dismutase 1 (SOD1). Mutant SOD1 A4V (alanine to valine) induces endoplasmic reticulum (ER) stress, which is increasingly implicated as a pathway to motor neuron degeneration and death in ALS. However, it remains unclear how ER stress is induced by mutant SOD1 A4V. Previous studies have established that it is induced early in pathophysiology and it precedes the formation of mutant SOD1 inclusions. SOD1 contains four cysteine residues, two of which form an intra-subunit disulphide bond involving Cys-57 and Cys-146. The remaining two cysteines, Cys-6 and Cys-111, remain unpaired and have been implicated in mutant SOD1 aggregation. In this study, we examined the relationship between the SOD1 A4V cysteine residues and aggregation, ER stress induction and toxicity. We report here that mutation of Cys-6 and Cys-111 in mutant SOD1 A4V, but not Cys-57 or Cys-146, ameliorates ER stress, inclusion formation and apoptosis in neuronal cell lines. These results imply that protein misfolding, induced by Cys-6 and Cys-111, is required for these pathological events in neuronal cells.





Similar content being viewed by others
Change history
11 June 2020
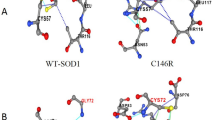
The original version of this article unfortunately contained an error in Fig. 3. The image shown for “C57S” was incorrect
References
Andersen PM, Nilsson P, Ala-Hurula V, Keränen M-L, Tarvainen I, Haltia T, Nilsson L, Binzer M, Forsgren L, Marklund SL (1995) Amyotrophic lateral sclerosis associated with homozygosity for an Asp90Ala mutation in CuZn-superoxide dismutase. Nat Genet 10(1):61–66
Anzai I, Tokuda E, Mukaiyama A, Akiyama S, Endo F, Yamanaka K, Misawa H, Furukawa Y (2017) A misfolded dimer of Cu/Zn-superoxide dismutase leading to pathological oligomerization in amyotrophic lateral sclerosis. Protein Sci 26(3):484–496
Arnesano F, Banci L, Bertini I, Martinelli M, Furukawa Y, O'Halloran TV (2004) The unusually stable quaternary structure of human Cu, Zn-superoxide dismutase 1 is controlled by both metal occupancy and disulfide status. J Biol Chem 279(46):47998–48003
Atkin JD, Farg MA, Soo KY, Walker AK, Halloran M, Turner BJ, Nagley P, Horne MK (2014) Mutant SOD 1 inhibits ER-Golgi transport in amyotrophic lateral sclerosis. J Neurochem 129(1):190–204
Banci L, Bertini I, Cramaro F, Del Conte R, Viezzoli MS (2002) The solution structure of reduced dimeric copper zinc superoxide dismutase: the structural effects of dimerization. Eur J Biochem 269(7):1905–1915
Brown RH, Al-Chalabi A (2017) Amyotrophic lateral sclerosis. N Engl J Med 377(2):162–172
Corcia P, Couratier P, Blasco H, Andres CR, Beltran S, Meininger V, Vourc'h P (2017) Genetics of amyotrophic lateral sclerosis. Rev Neurol 173(5):254–262. https://doi.org/10.1016/j.neurol.2017.03.030
Cozzolino M, Amori I, Pesaresi MG, Ferri A, Nencini M, Carrì MT (2008) Cysteine 111 affects aggregation and cytotoxicity of mutant Cu, Zn-superoxide dismutase associated with familial amyotrophic lateral sclerosis. J Biol Chem 283(2):866–874
Cudkowicz M, McKenna-Yasek D, Sapp P, Chin W, Geller B, Hayden D et al (1997) Epidemiology of mutations in superoxide dismutase in amyotrophic lateal sclerosis. Ann Neurol 41(2):210–221
Cummings BS, Schnellmann RG (2004) Measurement of cell death in mammalian cells. Curr Protoc Pharmacol, Chapter 12, https://doi.org/10.1002/0471141755.ph1208s25
Deng H-X, Shi Y, Furukawa Y, Zhai H, Fu R, Liu E et al (2006) Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci 103(18):7142–7147
Fujiwara N, Nakano M, Kato S, Yoshihara D, Ookawara T, Eguchi H, Taniguchi N, Suzuki K (2007) Oxidative modification to cysteine sulfonic acid of Cys111 in human copper-zinc superoxide dismutase. J Biol Chem 282(49):35933–35944
Furukawa Y, Fu R, Deng H-X, Siddique T, O'Halloran TV (2006) Disulfide cross-linked protein represents a significant fraction of ALS-associated Cu, Zn-superoxide dismutase aggregates in spinal cords of model mice. Proc Natl Acad Sci 103(18):7148–7153
Hand CK, Mayeux-Portas V, Khoris J, Briolotti V, Clavelou P, Camu W, Rouleau GA (2001) Compound heterozygous D90A and D96N SOD1 mutations in a recessive amyotrophic lateral sclerosis family. Ann Neurol 49(2):267–271
Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13(2):89–102
Hough MA, Grossmann JG, Antonyuk SV, Strange RW, Doucette PA, Rodriguez JA et al (2004) Dimer destabilization in superoxide dismutase may result in disease-causing properties: structures of motor neuron disease mutants. Proc Natl Acad Sci 101(16):5976–5981
Ito Y, Yamada M, Tanaka H, Aida K, Tsuruma K, Shimazawa M, Hozumi I, Inuzuka T, Takahashi H, Hara H (2009) Involvement of CHOP, an ER-stress apoptotic mediator, in both human sporadic ALS and ALS model mice. Neurobiol Dis 36(3):470–476
Johnston JA, Dalton MJ, Gurney ME, Kopito RR (2000) Formation of high molecular weight complexes of mutant cu, Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis. Proc Natl Acad Sci 97(23):12571–12576
Jonsson PA, Graffmo KS, Andersen PM, Brännström T, Lindberg M, Oliveberg M, Marklund SL (2005) Disulphide-reduced superoxide dismutase-1 in CNS of transgenic amyotrophic lateral sclerosis models. Brain 129(2):451–464
Karch CM, Borchelt DR (2008) A limited role for disulfide cross-linking in the aggregation of mutant SOD1 linked to familial amyotrophic lateral sclerosis. J Biol Chem 283(20):13528–13537
Karch CM, Prudencio M, Winkler DD, Hart PJ, Borchelt DR (2009) Role of mutant SOD1 disulfide oxidation and aggregation in the pathogenesis of familial ALS. Proc Natl Acad Sci 106(19):7774–7779
Kerman A, Liu H-N, Croul S, Bilbao J, Rogaeva E, Zinman L, Robertson J, Chakrabartty A (2010) Amyotrophic lateral sclerosis is a non-amyloid disease in which extensive misfolding of SOD1 is unique to the familial form. Acta Neuropathol 119(3):335–344
Keskin I, Forsgren E, Lehmann M, Andersen PM, Brännström T, Lange DJ et al (2018) The molecular pathogenesis of superoxide dismutase 1-linked ALS is promoted by low oxygen tension. bioRxiv:320283
Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23(21):7448–7459
Medinas DB, Rozas P, Traub FM, Woehlbier U, Brown RH, Bosco DA, Hetz C (2018) Endoplasmic reticulum stress leads to accumulation of wild-type SOD1 aggregates associated with sporadic amyotrophic lateral sclerosis. Proc Natl Acad Sci 115(32):8209–8214
Medinas DB, Cabral-Miranda F, Hetz C (2019) ER stress links aging to sporadic ALS. Aging (Albany NY) 11(1):5–6
Montibeller L, de Belleroche J (2018) Amyotrophic lateral sclerosis (ALS) and Alzheimer’s disease (AD) are characterised by differential activation of ER stress pathways: focus on UPR target genes. Cell Stress Chaperones 23(5):897–912
Nishitoh H, Kadowaki H, Nagai A, Maruyama T, Yokota T, Fukutomi H, Noguchi T, Matsuzawa A, Takeda K, Ichijo H (2008) ALS-linked mutant SOD1 induces ER stress-and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev 22(11):1451–1464
Niwa J-i, Yamada S-i, Ishigaki S, Sone J, Takahashi M, Katsuno M et al (2007) Disulfide bond mediates aggregation, toxicity, and ubiquitylation of familial amyotrophic lateral sclerosis-linked mutant SOD1. J Biol Chem 282(38):28087–28095
Parakh S, Atkin JD (2016) Protein folding alterations in amyotrophic lateral sclerosis. Brain Res 1648:633–649
Parakh S, Jagaraj CJ, Vidal M, Ragagnin AMG, Perri ER, Konopka A, Toth RP, Galper J, Blair IP, Thomas CJ, Walker AK, Yang S, Spencer DM, Atkin JD (2018) ERp57 is protective against mutant SOD1-induced cellular pathology in amyotrophic lateral sclerosis. Hum Mol Genet 27(8):1311–1331. https://doi.org/10.1093/hmg/ddy041
Perri ER, Thomas CJ, Parakh S, Spencer DM, Atkin JD (2015) The unfolded protein response and the role of protein disulfide isomerase in neurodegeneration. Front Cell Dev Biol 3:80. https://doi.org/10.3389/fcell.2015.00080
Rakhit R, Crow JP, Lepock JR, Kondejewski LH, Cashman NR, Chakrabartty A (2004) Monomeric Cu, Zn-superoxide dismutase is a common misfolding intermediate in the oxidation models of sporadic and familial amyotrophic lateral sclerosis. J Biol Chem 279(15):15499–15504
Renton AE, Chiò A, Traynor BJ (2014) State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 17(1):17–23
Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A et al (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362(6415):59–62
Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ (2006) Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol 4(11):e374. https://doi.org/10.1371/journal.pbio.0040374
Saeed M, Yang Y, Deng H, Hung W, Siddique N, Dellefave L et al (2009) Age and founder effect of SOD1 A4V mutation causing ALS. Neurology 72(19):1634–1639
Saxena S, Cabuy E, Caroni P (2009) A role for motoneuron subtype–selective ER stress in disease manifestations of FALS mice. Nat Neurosci 12(5):627–636
Shaffer A, Shapiro-Shelef M, Iwakoshi NN, Lee A-H, Qian S-B, Zhao H et al (2004) XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21(1):81–93
Soo KY, Atkin JD, Horne MK, Nagley P (2009) Recruitment of mitochondria into apoptotic signaling correlates with the presence of inclusions formed by amyotrophic lateral sclerosis-associated SOD1 mutations. J Neurochem 108(3):578–590
Soo KY, Halloran M, Sundaramoorthy V, Parakh S, Toth RP, Southam KA et al (2015) Rab1-dependent ER–Golgi transport dysfunction is a common pathogenic mechanism in SOD1, TDP-43 and FUS-associated ALS. Acta Neuropathol 130(5):679–697
Stathopulos P, Rumfeldt J, Scholz G, Irani R, Frey H, Hallewell R et al (2003) Cu/Zn superoxide dismutase mutants associated with amyotrophic lateral sclerosis show enhanced formation of aggregates in vitro. Proc Natl Acad Sci 100(12):7021–7026
Tadic V, Prell T, Lautenschlaeger J, Grosskreutz J (2014) The ER mitochondria calcium cycle and ER stress response as therapeutic targets in amyotrophic lateral sclerosis. Front Cell Neurosci 8:147
Tiwari A, Hayward LJ (2003) Familial amyotrophic lateral sclerosis mutants of copper/zinc superoxide dismutase are susceptible to disulfide reduction. J Biol Chem 278(8):5984–5992
Turner BJ, Atkin JD, Farg MA, Zang DW, Rembach A, Lopes EC, Patch JD, Hill AF, Cheema SS (2005) Impaired extracellular secretion of mutant superoxide dismutase 1 associates with neurotoxicity in familial amyotrophic lateral sclerosis. J Neurosci 25(1):108–117. https://doi.org/10.1523/JNEUROSCI.4253-04.2005
Walker AK, Farg MA, Bye CR, McLean CA, Horne MK, Atkin JD (2010) Protein disulphide isomerase protects against protein aggregation and is S-nitrosylated in amyotrophic lateral sclerosis. Brain 133(1):105–116
Wallis N, Lau CL, Farg MA, Atkin JD, Beart PM, O’Shea RD (2018) SOD1 mutations causing familial amyotrophic lateral sclerosis induce toxicity in astrocytes: evidence for bystander effects in a continuum of astrogliosis. Neurochem Res 43(1):157–170
Wang J, Xu G, Borchelt DR (2006) Mapping superoxide dismutase 1 domains of non-native interaction: roles of intra-and intermolecular disulfide bonding in aggregation. J Neurochem 96(5):1277–1288
Watanabe S, Nagano S, Duce J, Kiaei M, Li Q-X, Tucker SM et al (2007) Increased affinity for copper mediated by cysteine 111 in forms of mutant superoxide dismutase 1 linked to amyotrophic lateral sclerosis. Free Radic Biol Med 42(10):1534–1542
Xu C, Bailly-Maitre B, Reed JC (2005) Endoplasmic reticulum stress: cell life and death decisions. J Clin Investig 115(10):2656–2664
Zhang Y-J, Jansen-West K, Xu Y-F, Gendron TF, Bieniek KF, Lin W-L et al (2014) Aggregation-prone c9FTD/ALS poly (GA) RAN-translated proteins cause neurotoxicity by inducing ER stress. Acta Neuropathol 128(4):505–524
Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12(7):982–995
Funding
This work is supported by The Australian National Health and Medical Research Council Project grants (1006141, 1030513, 1086887 and 1124005); Dementia Teams Research Grant (1095215); Fight MND Foundation; and Motor Neuron Disease Research Institute of Australia. EP was supported by an Australian Postgraduate Award scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 12 kb).
Rights and permissions
About this article
Cite this article
Perri, E.R., Parakh, S., Vidal, M. et al. The Cysteine (Cys) Residues Cys-6 and Cys-111 in Mutant Superoxide Dismutase 1 (SOD1) A4V Are Required for Induction of Endoplasmic Reticulum Stress in Amyotrophic Lateral Sclerosis. J Mol Neurosci 70, 1357–1368 (2020). https://doi.org/10.1007/s12031-020-01551-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-020-01551-6