Abstract
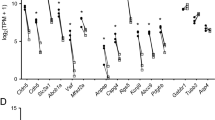
Angiogenesis is induced immediately after cerebral ischemia and plays a pivotal role in the strategy against ischemic injury. We hypothesized that the coordinated interaction between microvessels and neurons was altered immediately after stroke, and microvessels and neurons would show the temporal specificity of angiogenic gene profiles after cerebral ischemia. Microvessels and neurons were harvested in the ischemic penumbra of rat brain using the PixCell II laser capture microdissection (LCM) instrument. After RNA isolation, T7 and gene-specific primer RNA linear amplification were performed, and angiogenic functional grouping cDNA profiling was analyzed in LCM samples. cDNA microarray results showed there were 35 (36.46%) and 27 (28.13%) genes expression changes in the microvessels, while 25 (26.04%) and 31 (32.29%) genes were changed in the neurons at 2 h and 24 h after cerebral ischemia. Members of growth factors and receptors, cytokines and chemokines, adhesion molecules, matrix proteins, proteases, and inhibitors showed temporal and spatial differentiation in the microvessels and neurons after cerebral ischemia. This finding will help to understand the coordination and interaction between microvessels and neurons, and to elucidate the molecular mechanisms of angiogenesis after brain ischemic injury.






Similar content being viewed by others
References
Ashwal S, Tone B, Tian HR, Cole DJ, Pearce WJ (1998) Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion. Stroke 29:1037–1046 discussion 1047
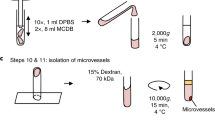
Ball HJ, McParland B, Driussi C, Hunt NH (2002) Isolating vessels from the mouse brain for gene expression analysis using laser capture microdissection. Brain Res Protoc 9:206–213
Conway EM, Collen D, Carmeliet P (2001) Molecular mechanisms of blood vessel growth. Cardiovasc Res 49:507–521
Datta S, Malhotra L, Dickerson R, Chaffee S, Sen CK, Roy S (2015) Laser capture microdissection: big data from small samples. Histol Histopathol 30:1255–1269
del Zoppo GJ, Hallenbeck JM (2000) Advances in the vascular pathophysiology of ischemic stroke. Thromb Res 98:73–81
Egashira Y, Suzuki Y, Azuma Y, Takagi T, Mishiro K, Sugitani S, Tsuruma K, Shimazawa M, Yoshimura S, Kashimata M, Iwama T, Hara H (2013) The growth factor progranulin attenuates neuronal injury induced by cerebral ischemia-reperfusion through the suppression of neutrophil recruitment. J Neuroinflammation 10:105
Espina V, Wulfkuhle JD, Calvert VS, VanMeter A, Zhou W, Coukos G, Geho DH, Petricoin EF 3rd, Liotta LA (2006) Laser-capture microdissection. Nat Protoc 1:586–603
Hatipoglu OF, Hirohata S, Cilek MZ, Ogawa H, Miyoshi T, Obika M, Demircan K, Shinohata R, Kusachi S, Ninomiya Y (2009) ADAMTS1 is a unique hypoxic early response gene expressed by endothelial cells. J Biol Chem 284:16325–16333
Hayashi T, Noshita N, Sugawara T, Chan PH (2003) Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab 23:166–180
Hayashi T, Deguchi K, Nagotani S, Zhang H, Sehara Y, Tsuchiya A, Abe K (2006) Cerebral ischemia and angiogenesis. Curr Neurovasc Res 3:119–129
Iruela-Arispe ML, Liska DJ, Sage EH, Bornstein P (1993) Differential expression of thrombospondin 1, 2, and 3 during murine development. Dev Dyn 197:40–56
Iruela-Arispe ML, Luque A, Lee N (2004) Thrombospondin modules and angiogenesis. Int J Biochem Cell Biol 36:1070–1078
Kamme F, Salunga R, Yu J, Tran DT, Zhu J, Luo L, Bittner A, Guo HQ, Miller N, Wan J, Erlander M (2003) Single-cell microarray analysis in hippocampus CA1: demonstration and validation of cellular heterogeneity. J Neurosci 23:3607–3615
Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM (1994) Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 25:1794–1798
Lapi D, Colantuoni A (2015) Remodeling of cerebral microcirculation after ischemia-reperfusion. J Vasc Res 52:22–31
Lin TN, Kim GM, Chen JJ, Cheung WM, He YY, Hsu CY (2003) Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke 34:177–186
Liu J, Wang Y, Akamatsu Y, Lee CC, Stetler RA, Lawton MT, Yang GY (2014) Vascular remodeling after ischemic stroke: mechanisms and therapeutic potentials. Prog Neurobiol 115:138–156
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Lu A, Tang Y, Ran R, Clark JF, Aronow BJ, Sharp FR (2003) Genomics of the periinfarction cortex after focal cerebral ischemia. J Cereb Blood Flow Metab 23:786–810
Lu XC, Williams AJ, Yao C, Berti R, Hartings JA, Whipple R, Vahey MT, Polavarapu RG, Woller KL, Tortella FC, Dave JR (2004) Microarray analysis of acute and delayed gene expression profile in rats after focal ischemic brain injury and reperfusion. J Neurosci Res 77:843–857
Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, Petit E, Risau W (2000) Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol 156:965–976
Matthews RT, Gary SC, Zerillo C, Pratta M, Solomon K, Arner EC, Hockfield S (2000) Brain-enriched hyaluronan binding (BEHAB)/brevican cleavage in a glioma cell line is mediated by a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) family member. J Biol Chem 275:22695–22703
Mojsilovic-Petrovic J, Nesic M, Pen A, Zhang W, Stanimirovic D (2004) Development of rapid staining protocols for laser-capture microdissection of brain vessels from human and rat coupled to gene expression analyses. J Neurosci Methods 133:39–48
Park JA, Choi KS, Kim SY, Kim KW (2003) Coordinated interaction of the vascular and nervous systems: from molecule- to cell-based approaches. Biochem Biophys Res Commun 311:247–253
Ren C, Yao Y, Han R, Huang Q, Li H, Wang B, Li S, Li M, Mao Y, Mao X, Xie L, Zhou L, Hu J, Ji X, Jin K (2018) Cerebral ischemia induces angiogenesis in the peri-infarct regions via Notch1 signaling activation. Exp Neurol 304:30–40
Sarabi AS, Shen H, Wang Y, Hoffer BJ, Backman CM (2008) Gene expression patterns in mouse cortical penumbra after focal ischemic brain injury and reperfusion. J Neurosci Res 86:2912–2924
Schwarz DA, Barry G, Mackay KB, Manu F, Naeve GS, Vana AM, Verge G, Conlon PJ, Foster AC, Maki RA (2002) Identification of differentially expressed genes induced by transient ischemic stroke. Brain Res Mol Brain Res 101:12–22
Seto SW, Chang D, Jenkins A, Bensoussan A, Kiat H (2016) Angiogenesis in ischemic stroke and angiogenic effects of Chinese herbal medicine. J Clin Med 5:56. https://doi.org/10.3390/jcm5060056
Tian YF, Zhang PB, Xiao XL, Zhang JS, Zhao JJ, Kang QY, Chen XL, Qiu F, Liu Y (2007) The quantification of ADAMTS expression in an animal model of cerebral ischemia using real-time PCR. Acta Anaesthesiol Scand 51:158–164
Vazquez F, Hastings G, Ortega MA, Lane TF, Oikemus S, Lombardo M, Iruela-Arispe ML (1999) METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem 274:23349–23357
Westling J, Gottschall PE, Thompson VP, Cockburn A, Perides G, Zimmermann DR, Sandy JD (2004) ADAMTS4 (aggrecanase-1) cleaves human brain versican V2 at Glu405-Gln406 to generate glial hyaluronate binding protein. Biochem J 377:787–795
Yin KJ, Hamblin M, Chen YE (2015) Angiogenesis-regulating microRNAs and ischemic stroke. Curr Vasc Pharmacol 13:352–365
Acknowledgements
We thank the Laboratory Animal Research Center at Xi’an Jiaotong College University of Medicine for their animal care and support, and Xiaoge Zhao for slice cutting.
Funding
This research was supported by the National Natural Science Foundation of China (31371501), Natural Science Foundation of Shaanxi Province (2017JM8047, 2018JM7066), and the Fundamental Research Funds for the Central Universities (GK201702006), Shaanxi Normal University.
Author information
Authors and Affiliations
Contributions
TY and LY conceived and designed the experiments. ZP and ZJ conducted pMCAO rat models. TY, DY, CX, and ZJ performed LCM experiments. FT, FJ, and SM did immunochemistry experiments. TY conducted qPCR experiments. TY wrote the article with the insightful advice from FA. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplementary Figure 1
Representative coronal sections of rat brain stained with TTC at 24 h after pMCAO. Infarct brain tissue was in a white or pink color embedded in the healthy brain tissue of deep red color with TTC stain. The infarct size ranges from 30.3 to 38.6% in our study. (PNG 706 kb)
Supplementary Figure 2
The expression of Cd31/ Pecam1, NeuN/ Rbfox3, and Gfap mRNA in LCM-captured microvessels and neurons. M: Marker. (PNG 259 kb)
Supplementary Table 1
Genes that increased in microvessels at 2 h after ischemia (27 genes) (DOCX 18 kb)
Supplementary Table 2
Genes that increased in microvessels at 24 h after ischemia (23 genes) (DOCX 17 kb)
Supplementary Table 3
Genes that decreased in microvessels at 2 h after ischemia (8 genes) (DOCX 14 kb)
Supplementary Table 4
Genes that decreased in microvessels at 24 h after ischemia (4 genes) (DOCX 14 kb)
Supplementary Table 5
Genes that increased in neurons at 2 h after ischemia (16 genes) (DOCX 17 kb)
Supplementary Table 6
Genes that increased in neurons at 24 h after ischemia (26 genes) (DOCX 18 kb)
Supplementary Table 7
Genes that decreased in neurons at 2 h after ischemia (9 genes) (DOCX 17 kb)
Supplementary Table 8
Genes that decreased in neurons at 24 h after ischemia (5 genes) (DOCX 15 kb)
Supplementary Table 9
Genes that highly expressed in microvessels compared to neurons at control group (14 genes) (DOCX 20 kb)
Supplementary Table 10
Genes that highly expressed in neurons compared to microvessels at control group (9 genes) (DOCX 14 kb)
Supplementary Table 11
Genes that highly expressed in microvessels compared to neurons at 2 h after ischemia (17 genes) (DOCX 18 kb)
Supplementary Table 12
Genes that highly expressed in neurons compared to microvessels at 2 h after ischemia (10 genes) (DOCX 15 kb)
Supplementary Table 13
Genes that highly expressed in microvessels compared to neurons at 24 h after ischemia (16 genes) (DOCX 16 kb)
Supplementary Table 14
Genes that highly expressed in neurons compared to microvessels at 24 h after ischemia (10 genes) (DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Tian, Y., Di, Y., Zhang, J. et al. Angiogenic Gene Profiles in Laser-Microdissected Microvessels and Neurons from Ischemic Penumbra of Rat Brain. J Mol Neurosci 67, 643–653 (2019). https://doi.org/10.1007/s12031-019-01270-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-019-01270-7