Abstract
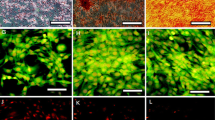
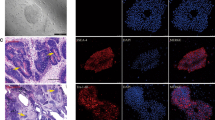
Cell therapy and stem cell transplantation strategies have provided potential therapeutic approaches for the treatment of neurological disorders. Adipose-derived mesenchymal stem cells (ADMSCs) are abundant adult stem cells with low immunogenicity, which can be used for allogeneic cell replacement therapies. Differentiation of ADMSCs into acetylcholine-secreting motoneurons (MNs) is a promising treatment for MN diseases, such as spinal muscular atrophy (SMA), which is associated with the level of SMN1 gene expression. The SMN2 gene plays an important role in MN disorders, as it can somewhat compensate for the lack of SMN1 expression in SMA patients. Although the differentiation potential of ADMSCs into MNs has been previously established, overexpression of SMN2 gene in a shorter period with a longer survival has yet to be elucidated. Ponasterone A (PNA), an ecdysteroid hormone activating the PI3K/Akt pathway, was studied as a new steroid to promote SMN2 overexpression in MNs differentiated from ADMSCs. After induction with retinoic acid, sonic hedgehog, forskolin, and PNA, MN phenotypes were differentiated from ADMSCs, and immunochemical staining, specific for β-tubulin, neuron-specific enolase, and choline acetyltransferase, was performed. Also, the results of real-time PCR assay indicated nestin, Pax6, Nkx2.2, Hb9, Olig2, and SMN2 expression in the differentiated cells. After 2 weeks of treatment, cultures supplemented with PNA showed a longer survival and a 1.2-fold increase in the expression of SMN2 (an overall 5.6-fold increase; *P ≤ 0.05), as confirmed by the Western blot analysis. The PNA treatment increased the levels of ChAT, Isl1, Hb9, and Nkx2 expression in MN-like cells. Our findings highlight the role of PNA in the upregulation of SMN2 genes from MSC-derived MN-like cells, which may serve as a potential candidate in cellular therapy for SMA patients.






Similar content being viewed by others
References
Allodi I, Comley L, Nichterwitz S, Nizzardo M, Simone C, Benitez JA, Cao M, Corti S, Hedlund E (2016) Differential neuronal vulnerability identifies IGF-2 as a protective factor in ALS. Sci Rep 6:25960
Also-Rallo E, Alías L, Martínez-Hernández R, Caselles L, Barceló MJ, Baiget M, Bernal S, Tizzano EF (2011) Treatment of spinal muscular atrophy cells with drugs that upregulate SMN expression reveals inter- and intra-patient variability. Eur J Hum Genet 19(10):1059–1065
Amirkhani MA, Mohseni R, Soleimani M, Shoae-Hassani A, Nilforoushzadeh MA (2016) A rapid sonication based method for preparation of stromal vascular fraction and mesenchymal stem cells from fat tissue. Bioimpacts 6(2):99–104
Ashcroft M, Stephens RM, Hallberg B, Downward J, Kaplan DR (1999) The selective and inducible activation of endogenous PI3-kinase in PC12 cells results in efficient NGF-mediated survival but defective neurite outgrowth. Oncogene 18:4586–4597
Bagher Z et al (2015) Differentiation of Wharton’s jelly-derived mesenchymal stem cells into motor neuron-like cells on three-dimensional collagen-grafted nanofibers. Mol Neurobiol 53:2397–2408
Bahrami N, Bayat M, Mohamadnia A, Khakbiz M, Yazdankhah M, Ai J, Ebrahimi-Barough S (2017) Purmorphamine as a Shh signaling activator small molecule promotes motor neuron differentiation of mesenchymal stem cells cultured on nanofibrous PCL scaffold. Mol Neurobiol 54(7):5668–5675
Bertini E, Mercuri E (2018) Motor neuron disease: a prospective natural history study of type 1 spinal muscular atrophy. Nat Rev Neurol 14(4):197–198
Biondi O, Branchu J, Sanchez G, Lancelin C, Deforges S, Lopes P, Pariset C, Lecolle S, Cote J, Chanoine C, Charbonnier F (2010) In vivo NMDA receptor activation accelerates motor unit maturation, protects spinal motor neurons, and enhances SMN2 gene expression in severe spinal muscular atrophy mice. J Neurosci 30(34):11288–11299
Branchu J, Biondi O, Chali F, Collin T, Leroy F, Mamchaoui K, Makoukji J, Pariset C, Lopes P, Massaad C, Chanoine C, Charbonnier F (2013) Shift from extracellular signal-regulated kinase to AKT/cAMP response element-binding protein pathway increases survival-motor-neuron expression in spinal-muscular-atrophy-like mice and patient cells. J Neurosci 33(10):4280–4294
Brichta L, Hofmann Y, Hahnen E, Siebzehnrubl FA, Raschke H, Blumcke I, Eyupoglu IY, Wirth B (2003) Valproic acid increases the SMN2 protein level: a well-known drug as a potential therapy for spinal muscular atrophy. Hum Mol Genet 12(19):2481–2489
Catalan RE, Aragones MD, Godoy JE, Martinez AM (1984) Ecdysterone induces acetylcholinesterase in mammalian brain. Comp Biochem Physiol C 78(1):193–195
Chen YC, Chang JG, Jong YJ, Liu TY, Yuo CY (2015) High expression level of Tra2-beta1 is responsible for increased SMN2 exon 7 inclusion in the testis of SMA mice. PLoS One 10(3):e0120721
Constantino S, Santos R, Gisselbrecht S, Gouilleux F (2001) The ecdysteroid inducible gene expression system: unexpected effects of muristerone A and ponasterone A on cytokine signalling in mammalian cells. Eur Cytokine Netw 12:365–367
Darvishi M, Tiraihi T, Mesbah-Namin SA, Delshad AR, Taheri T (2017a) Motor neuron transdifferentiation of neural stem cell from adipose-derived stem cell characterized by differential gene expression. Cell Mol Neurobiol 37(2):275–289
Darvishi M et al (2017b) Motor neuron transdifferentiation of neural stem cell from adipose-derived stem cell characterized by differential gene expression. Cellular and Molecular Neurobiology 37(2):275–289
De Sanctis R et al (2018) Clinical phenotypes and trajectories of disease progression in type 1 spinal muscular atrophy. Neuromuscul Disord 28(1):24–28
Dominici M, le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317
Du ZW et al (2015) Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat Commun 6:6626
Ebrahimi-Barough S, Hoveizi E, Yazdankhah M, Ai J, Khakbiz M, Faghihi F, Tajerian R, Bayat N (2017) Inhibitor of PI3K/Akt signaling pathway small molecule promotes motor neuron differentiation of human endometrial stem cells cultured on electrospun biocomposite polycaprolactone/collagen scaffolds. Mol Neurobiol 54(4):2547–2554
Faghihi F, Mirzaei E, Ai J, Lotfi A, Sayahpour FA, Barough SE, Joghataei MT (2016) Differentiation potential of human chorion-derived mesenchymal stem cells into motor neuron-like cells in two- and threedimensional culture systems. Mol Neurobiol 53(3):1862–1872. https://doi.org/10.1007/s12035-015-9129-y
Gavrilina TO, McGovern VL, Workman E, Crawford TO, Gogliotti RG, DiDonato CJ, Monani UR, Morris GE, Burghes AHM (2008) Neuronal SMN expression corrects spinal muscular atrophy in severe SMA mice while muscle-specific SMN expression has no phenotypic effect. Hum Mol Genet 17:1063–1075
Guo JS, Zeng YS, Li HB, Huang WL, Liu RY, Li XB, Ding Y, Wu LZ, Cai DZ (2007) Cotransplant of neural stem cells and NT-3 gene modified Schwann cells promote the recovery of transected spinal cord injury. Spinal Cord 45(1):15–24
Hao le T et al (2012) Survival motor neuron affects plastin 3 protein levels leading to motor defects. J Neurosci 32(15):5074–5084
Haque A, Polcyn R, Matzelle D, Banik NL (2018) New insights into the role of Neuron-Specific Enolase in neuro-inflammation, neurodegeneration, and neuroprotection. Brain Sci 18;8(2). https://doi.org/10.3390/brainsci8020033
Hayashi H, Tsuchiya Y, Nakayama K, Satoh T, Nishida E (2008) Down-regulation of the PI3-kinase/Akt pathway by ERK MAP kinase in growth factor signaling. Genes Cells 13(9):941–947
Hollis ER, Jamshidi P, Low K, Blesch A, Tuszynski MH (2009) Induction of corticospinal regeneration by lentiviral trkB-induced Erk activation. Proc Natl Acad Sci U S A 106(17):7215–7220
Groen JNE et al (2018) Temporal and tissue-specific variability of SMN protein levels in mouse models of spinal muscular atrophy. Hum Mol Genet 27(16):2851–2862
Kostova FV, Williams VC, Heemskerk J, Iannaccone S, DiDonato C, Swoboda K, Maria BL (2007) Spinal muscular atrophy: classification, diagnosis, management, pathogenesis, and future research directions. J Child Neurol 22(8):926–945
Lafont R, Dinan L (2003) Practical uses for ecdysteroids in mammals including humans: an update. J Insect Sci 3:7
Le TT et al (2000) The survival motor neuron (SMN) protein: effect of exon loss and mutation on protein localization. Neurogenetics 3(1):7–16
Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, le Paslier D, Frézal J, Cohen D, Weissenbach J, Munnich A, Melki J (1995) Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80(1):155–165
Liqing Y, Jia G, Jiqing C, Ran G, Fei C, Jie K, Yanyun W, Cheng Z (2011) Directed differentiation of motor neuron cell-like cells from human adipose-derived stem cells in vitro. Neuroreport 22(8):370–373
Liu X, Li D, Jiang D, Fang Y (2013) Acetylcholine secretion by motor neuron-like cells from umbilical cord mesenchymal stem cells. Neural Regen Res 8:2086–2092
López-Carballo G, Moreno L, Masiá S, Pérez P, Barettino D (2002) Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway by retinoic acid is required for neural differentiation of SH-SY5Y human neuroblastoma cells. J Biol Chem 277:25297–25304
Lorson CL, Rindt H, Shababi M (2010) Spinal muscular atrophy: mechanisms and therapeutic strategies. Hum Mol Genet 19(R1):R111–R118
Lunn JS, Sakowski SA, Federici T, Glass JD, Boulis NM, Feldman EL (2011) Stem cell technology for the study and treatment of motor neuron diseases. Regen Med 6(2):201–213
Maruyama R, Touznik A, Yokota T (2018) Evaluation of exon inclusion induced by splice switching antisense oligonucleotides in SMA patient fibroblasts. J Vis Exp 11(135). https://doi.org/10.3791/57530
Monani UR, Coovert DD, Burghes AH (2000) Animal models of spinal muscular atrophy. Hum Mol Genet 9(16):2451–2457
Noureddini M, Verdi J, Mortazavi-Tabatabaei SA, Sharif S, Azimi A, Keyhanvar P, Shoae-Hassani A (2012) Human endometrial stem cell neurogenesis in response to NGF and bFGF. Cell Biol Int 36(10):961–966
Oehme I, Bosser S, Zornig M (2006) Agonists of an ecdysone-inducible mammalian expression system inhibit Fas Ligand- and TRAIL-induced apoptosis in the human colon carcinoma cell line RKO. Cell Death Differ 13(2):189–201
Oh S, Huang X, Liu J, Litingtung Y, Chiang C (2009) Shh and Gli3 activities are required for timely generation of motor neuron progenitors. Dev Biol 331(2):261–269
Ojeda L, Gao J, Hooten KG, Wang E, Thonhoff JR, Dunn TJ, Gao T, Wu P (2011) Critical role of PI3K/Akt/GSK3beta in motoneuron specification from human neural stem cells in response to FGF2 and EGF. PLoS One 6(8):e23414
Okada M, Ishihara K, Sasa M, Izumi R, Yajin K, Harada Y (1998) Enhancement of GABA-mediated inhibition of rat medial vestibular nucleus neurons by the neurosteroid 20-hydroxyecdysone. Acta Otolaryngol 118(1):11–16
Rahmani A et al (2013) Neurogenesis and increase in differentiated neural cell survival via phosphorylation of Akt1 after fluoxetine treatment of stem cells. Biomed Res Int 2013:582526
Richardson RC (2018) Cost-effectiveness of Nusinersen for spinal muscular atrophy. JAMA Pediatr 172:701
Riddiford LM, Cherbas P, Truman JW (2000) Ecdysone receptors and their biological actions. Vitam Horm 60:1–73
Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J (1997) Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell 89(3):457–467
Seo J, Howell MD, Singh NN, Singh RN (2013) Spinal muscular atrophy: an update on therapeutic progress. Biochim Biophys Acta 1832(12):2180–2190
Shoae-Hassani A, Behfar M, Mortazavi-Tabatabaei SA, Ai J, Mohseni R, Hamidieh AA (2017) Natural killer cells from the subcutaneous adipose tissue underexpress the NKp30 and NKp44 in obese persons and are less active against major histocompatibility complex class I non-expressing neoplastic cells. Front Immunol 8:1486
Sproule DM, Kaufmann P (2010) Therapeutic developments in spinal muscular atrophy. Ther Adv Neurol Disord 3(3):173–185
Subramanian C, Opipari AW, Castle VP, Kwok RPS (2005) Histone deacetylase inhibition induces apoptosis in neuroblastoma. Cell Cycle 4(12):1741–1743
Thonhoff JR, Ojeda L, Wu P (2009) Stem cell-derived motor neurons: applications and challenges in amyotrophic lateral sclerosis. Curr Stem Cell Res Ther 4(3):178–199
Tsujiyama S, Ujihara H, Ishihara K, Sasa M (1995) Potentiation of GABA-induced inhibition by 20-hydroxyecdysone, a neurosteroid, in cultured rat cortical neurons. Jpn J Pharmacol 68(1):133–136
Turner BJ, Alfazema N, Sheean RK, Sleigh JN, Davies KE, Horne MK, Talbot K (2014) Overexpression of survival motor neuron improves neuromuscular function and motor neuron survival in mutant SOD1 mice. Neurobiol Aging 35(4):906–915
Verdi J, Mortazavi-Tabatabaei SA, Sharif S, Verdi H, Shoae-Hassani A (2014a) Citalopram increases the differentiation efficacy of bone marrow mesenchymal stem cells into neuronal-like cells. Neural Regen Res 9(8):845–850
Verdi J, Sharif S, Banafshe HR, Shoae-Hassani A (2014b) Sertraline increases the survival of retinoic acid induced neuronal cells but not glial cells from human mesenchymal stem cells. Cell Biol Int 38(8):901–909
Wirth B, Brichta L, Schrank B, Lochmüller H, Blick S, Baasner A, Heller R (2006) Mildly affected patients with spinal muscular atrophy are partially protected by an increased SMN2 copy number. Hum Genet 119(4):422–428
Wu CY et al (2012) Efficient differentiation of mouse embryonic stem cells into motor neurons. J Vis Exp 9(64):e3813
Yang YM, Gupta SK, Kim KJ, Powers BE, Cerqueira A, Wainger BJ, Ngo HD, Rosowski KA, Schein PA, Ackeifi CA, Arvanites AC, Davidow LS, Woolf CJ, Rubin LL (2013) A small molecule screen in stem-cell-derived motor neurons identifies a kinase inhibitor as a candidate therapeutic for ALS. Cell Stem Cell 12(6):713–726
Zerres K, Rudnik-Schöneborn S, Forrest E, Lusakowska A, Borkowska J, Hausmanowa-Petrusewicz I (1997) A collaborative study on the natural history of childhood and juvenile onset proximal spinal muscular atrophy (type II and III SMA): 569 patients. J Neurol Sci 146(1):67–72
Zhang Z, Cai L, Zhou X, Su C, Xiao F, Gao Q, Luo H (2015) Methyl 3,4-dihydroxybenzoate promote rat cortical neurons survival and neurite outgrowth through the adenosine A2a receptor/PI3K/Akt signaling pathway. Neuroreport 26:367–373
Zheleznyakova GY, Kiselev AV, Vakharlovsky VG, Rask-Andersen M, Chavan R, Egorova AA, Schiöth HB, Baranov VS (2011) Genetic and expression studies of SMN2 gene in Russian patients with spinal muscular atrophy type II and III. BMC Med Genet 12:96
Zhou J, Du T, Li B, Rong Y, Verkhratsky A, Peng L (2015) Crosstalk between MAPK/ERK and PI3K/AKT signal pathways during brain ischemia/reperfusion. ASN Neuro 6;7(5). https://doi.org/10.1177/1759091415602463
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The fat tissue was obtained after proper consenting process and approvals from Tehran University of Medical Sciences ethics committee.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohseni, R., Ashrafi, M.R., Ai, J. et al. Overexpression of SMN2 Gene in Motoneuron-Like Cells Differentiated from Adipose-Derived Mesenchymal Stem Cells by Ponasterone A. J Mol Neurosci 67, 247–257 (2019). https://doi.org/10.1007/s12031-018-1232-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-018-1232-x