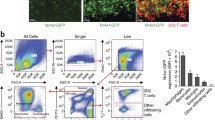
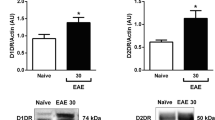
Abstract
The sympathetic nervous system (SNS) serves to maintain homeostasis of vital organ systems throughout the body, and its dysfunction plays a major role in human disease. The SNS also links the central nervous system to the immune system during different types of stress via innervation of the lymph nodes, spleen, thymus, and bone marrow. Previous studies have shown that pituitary adenylate cyclase-activating polypeptide (PACAP, gene name adcyap1) exhibits anti-inflammatory properties in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis. Because PACAP is known to regulate SNS function, we hypothesized that part of the immunoprotective action of PACAP is due to its neuromodulatory effects on sympathetic neurons. To examine this, we used an inducible, targeted approach to conditionally disrupt not only the PACAP-preferring PAC1 receptor gene (adcyap1r1) in dopamine β-hydroxylase-expressing cells, which includes postganglionic sympathetic neurons, but also catecholaminergic neurons in the brain and adrenomedullary chromaffin cells. In contrast to our previous EAE studies using PACAP global knockout mice which developed severe and prolonged EAE, we found that mice with conditional loss of PAC1 receptors in catecholaminergic cells developed a delayed time course of EAE with reduced helper T cell type 1 (Th1) and Th17 and enhanced Th2 cell polarization. At later time points, similar to mice with global PACAP loss, mice with conditional loss of PAC1 exhibited more severe clinical disease than controls. The latter was associated with a reduction in the abundance of thymic regulatory T cells (Tregs). These studies indicate that PAC1 receptor signaling acts in catecholaminergic cells in a time-dependent manner. At early stages of disease development, it enhances the ability of the SNS to polarize the Th response towards a more inflammatory state. Then, after disease is established, it enhances the ability of the SNS to dampen the inflammatory response via Tregs. The lack of concordance in results between global PACAP KO mice and mice with the PAC1 deletion targeted to catecholaminergic cells during early EAE may be explained by the fact that PACAP acts to regulate inflammation via multiple receptor subtypes and multiple targets, including inflammatory cells.






Similar content being viewed by others
Change history
26 October 2018
The original version of this article unfortunately contained mistakes. The captured article title and corresponding author were incorrect.
References
Abad C, Tan Y-V, Lopez R, Nobuta H, Dong H, Phan P, Feng J-M, Campagnoni AT, Waschek JA (2010) Vasoactive intestinal peptide loss leads to impaired CNS parenchymal T-cell infiltration and resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci 107:19555–19560
Abad C, Jayaram B, Becquet L, Wang Y, O’Dorisio MS, Waschek JA, Tan Y-V (2016) VPAC1 receptor (Vipr1)-deficient mice exhibit ameliorated experimental autoimmune encephalomyelitis, with specific deficits in the effector stage. J Neuroinflammation 13:169
Beaudet MM, Braas KM, May V (1998) Pituitary adenylate cyclase activating polypeptide (PACAP) expression in sympathetic preganglionic projection neurons to the superior cervical ganglion. J Neurobiol 36:325–336
Bellinger DL, Millar BA, Perez S, Carter J, Wood C, ThyagaRajan S, Molinaro C, Lubahn C, Lorton D (2008) Sympathetic modulation of immunity: relevance to disease. Cell Immunol 252:27–56
Braas KM, & May V (1999) Pituitary Adenylate Cyclase-activating Polypeptides Directly Stimulate Sympathetic Neuron Neuropeptide Y Release through PAC1 Receptor Isoform Activation of Specific Intracellular Signaling Pathways. J Biol Chem 274:27702–27710
Brifault C, Gras M, Liot D, May V, Vaudry D, Wurtz O (2014) Delayed pituitary adenylate cyclase-activating polypeptide delivery after brain stroke improves functional recovery by inducing M2 microglia/macrophage polarization. Stroke. https://doi.org/10.1161/STROKEAHA.114.006864
Chen W-H, Tzeng S-F (2005) Pituitary adenylate cyclase-activating polypeptide prevents cell death in the spinal cord with traumatic injury. Neurosci Lett 384:117–121
Constantinescu CS, Farooqi N, O’Brien K, Gran B (2011) Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol 164:1079–1106
Damsker JM, Hansen AM, Caspi RR (2010) Th1 and Th17 cells. Ann N Y Acad Sci 1183:211–221
Dejda A, Jolivel V, Bourgault S, Seaborn T, Fournier A, Vaudry H, Vaudry D (2008) Inhibitory effect of PACAP on caspase activity in neuronal apoptosis: a better understanding towards therapeutic applications in neurodegenerative diseases. J Mol Neurosci MN 36:26–37
Delgado M (2004) VIP/PACAP preferentially attract Th2 effectors through differential regulation of chemokine production by dendritic cells. FASEB J 18:1453–1455
Diane A, Nikolic N, Rudecki AP, King SM, Bowie DJ, & Gray SL (2014) PACAP is essential for the adaptive thermogenic response of brown adipose tissue to cold exposure. J Endocrinol 222:327–339
DiCicco-Bloom E, Deutsch PJ, Maltzman J, Zhang J, Pintar JE, Zheng J, Friedman WF, Zhou X, Zaremba T (2000) Autocrine expression and ontogenetic functions of the PACAP ligand/receptor system during sympathetic development. Dev Biol 219:197–213
Ebbinghaus M, Gajda M, Boettger MK, Schaible H-G, Bräuer R (2012) The anti-inflammatory effects of sympathectomy in murine antigen-induced arthritis are associated with a reduction of Th1 and Th17 responses. Ann Rheum Dis 71:253–261
Gijbels K, Brocke S, Abrams JS, Steinman L (1995) Administration of neutralizing antibodies to interleukin-6 (IL-6) reduces experimental autoimmune encephalomyelitis and is associated with elevated levels of IL-6 bioactivity in central nervous system and circulation. Mol Med 1:795–805
Härle P, Möbius D, Carr DJJ, Schölmerich J, Straub RH (2005) An opposing time-dependent immune-modulating effect of the sympathetic nervous system conferred by altering the cytokine profile in the local lymph nodes and spleen of mice with type II collagen-induced arthritis. Arthritis Rheum 52:1305–1313
Jamen F, Persson K, Bertrand G, Rodriguez-Henche N, Puech R, Bockaert J, Ahrén B, Brabet P (2000) PAC1 receptor-deficient mice display impaired insulinotropic response to glucose and reduced glucose tolerance. J Clin Invest 105:1307–1315
Kenney M, Ganta C (2014) Autonomic nervous system and immune system interactions. Compr Physiol 4:1177–1200
Khan RS, Fonseca-Kelly Z, Callinan C, Zuo L, Sachdeva MM, Shindler KS (2012) SIRT1 activating compounds reduce oxidative stress and prevent cell death in neuronal cells. Front Cell Neurosci 6. https://doi.org/10.3389/fncel.2012.00063
Kohm AP, Carpentier PA, Miller SD (2003) Regulation of experimental autoimmune encephalomyelitis (EAE) by CD4+CD25+ regulatory T cells. Novartis Found Symp 252:45–52 discussion 52-54, 106–114
Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Bäckström BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK (2007) Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med 13:423–431
Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, Kuchroo VK, Oukka M (2008) IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci 105:18460–18465
Kwon H-S, Lim HW, Wu J, Schnolzer M, Verdin E, Ott M (2012) Three novel acetylation sites in the Foxp3 transcription factor regulate the suppressive activity of regulatory T cells. J Immunol 188:2712–2721
Lafaille JJ (1998) The role of helper T cell subsets in autoimmune diseases. Cytokine Growth Factor Rev 9:139–151
Lee EH, Seo SR (2014) Neuroprotective roles of pituitary adenylate cyclase-activating polypeptide in neurodegenerative diseases. BMB Rep 47:369–375
Lim HW, Kang SG, Ryu JK, Schilling B, Fei M, Lee IS, Kehasse A, Shirakawa K, Yokoyama M, Schnölzer M, Kasler HG, Kwon HS, Gibson BW, Sato H, Akassoglou K, Xiao C, Littman DR, Ott M, Verdin E (2015) SIRT1 deacetylates RORγt and enhances Th17 cell generation. J Exp Med 212:607–617
Lin JT, Martin SL, Xia L, Gorham JD (2005) TGF-beta 1 uses distinct mechanisms to inhibit IFN-gamma expression in CD4+ T cells at priming and at recall: differential involvement of Stat4 and T-bet. J Immunol 1950(174):5950–5958
Lu N, Zhou R, DiCicco-Bloom E (1998) Opposing mitogenic regulation by PACAP in sympathetic and cerebral cortical precursors correlates with differential expression of PACAP receptor (PAC1-R) isoforms. J Neurosci Res 53:651–662
McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ (2007) TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat Immunol 8:1390–1397
Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH (1989) Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 164:567–574
Nance DM, Sanders VM (2007) Autonomic innervation and regulation of the immune system (1987-2007). Brain Behav Immun 21:736–745
Nimmagadda VK, Bever CT, Vattikunta NR, Talat S, Ahmad V, Nagalla NK, Trisler D, Judge SIV, Royal W, Chandrasekaran K et al. (2013) Overexpression of SIRT1 protein in neurons protects against experimental autoimmune encephalomyelitis through activation of multiple SIRT1 targets. J Immunol 190(9):4595–4607
Okuda Y, Sakoda S, Fujimura H, Saeki Y, Kishimoto T, Yanagihara T (1999) IL-6 plays a crucial role in the induction phase of myelin oligodendrocyte glucoprotein 35-55 induced experimental autoimmune encephalomyelitis. J Neuroimmunol 101:188–196
Olivas A, Gardner RT, Wang L, Ripplinger CM, Woodward WR, Habecker BA (2016) Myocardial infarction causes transient cholinergic transdifferentiation of cardiac sympathetic nerves via gp130. J Neurosci 36:479–488
Otto C (2004) Pulmonary hypertension and right heart failure in pituitary adenylate cyclase-activating polypeptide type I receptor-deficient mice. Circulation 110:3245–3251
Pettersson LME, Heine T, Verge VMK, Sundler F, Danielsen N (2004) PACAP mRNA is expressed in rat spinal cord neurons. J Comp Neurol 471:85–96
Samoilova EB, Horton JL, Hilliard B, Liu TS, Chen Y (1998) IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J. Immunol 1950(161):6480–6486
Sanders VM, Kasprowicz DJ, Swanson-Mungerson MA, Podojil JR, Kohm AP (2003) Adaptive immunity in mice lacking the beta(2)-adrenergic receptor. Brain Behav Immun 17:55–67
Seaborn T, Masmoudi-Kouli O, Fournier A, Vaudry H, Vaudry D (2011) Protective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) against apoptosis. Curr Pharm Des 17:204–214
Shadiack AM, Sun Y, Zigmond RE (2001) Nerve growth factor antiserum induces axotomy-like changes in neuropeptide expression in intact sympathetic and sensory neurons. J Neurosci 21:363–371
Simmons SB, Pierson ER, Lee SY, Goverman JM (2013) Modeling the heterogeneity of multiple sclerosis in animals. Trends Immunol 34:410–422
Sternberg Z (2016) Promoting sympathovagal balance in multiple sclerosis; pharmacological, non-pharmacological, and surgical strategies. Autoimmun Rev 15:113–123
Stubbusch J, Majdazari A, Schmidt M, Schütz G, Deller T, Rohrer H (2011) Generation of the tamoxifen-inducible DBH-Cre transgenic mouse line DBH-CT. Genes 2000(49):935–941
Tan Y-V, Abad C, Lopez R, Dong H, Liu S, Lee A, Gomariz RP, Leceta J, Waschek JA (2009) Pituitary adenylyl cyclase-activating polypeptide is an intrinsic regulator of Treg abundance and protects against experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 106:2012–2017
Tan Y-V, Abad C, Wang Y, Lopez R, Waschek JA (2013) Pituitary adenylate cyclase activating peptide deficient mice exhibit impaired thymic and extrathymic regulatory T cell proliferation during EAE. PLoS One 8:e61200
Tan Y-V, Abad C, Wang Y, Lopez R, Waschek JA (2015) VPAC2 (vasoactive intestinal peptide receptor type 2) receptor deficient mice develop exacerbated experimental autoimmune encephalomyelitis with increased Th1/Th17 and reduced Th2/Treg responses. Brain Behav Immun 44:167–175
Tsarovina K, Reiff T, Stubbusch J, Kurek D, Grosveld FG, Parlato R, Schütz G, Rohrer H (2010) The Gata3 transcription factor is required for the survival of embryonic and adult sympathetic neurons. J Neurosci 30:10833–10843
van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YYJ, Beekman JM, van Beekum O, Brenkman AB, Hijnen D-J, Mutis T, Kalkhoven E, Prakken BJ, Coffer PJ (2010) Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood 115:965–974
Veldhoen M, Hocking RJ, Flavell RA, Stockinger B (2006) Signals mediated by transforming growth factor-β initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol 7:1151–1156
Waschek J (2013) VIP and PACAP: neuropeptide modulators of CNS inflammation, injury, and repair: VIP and PACAP in neural injury. Br J Pharmacol 169:512–523
Zhang X, Koldzic DN, Izikson L, Reddy J, Nazareno RF, Sakaguchi S, Kuchroo VK, Weiner HL (2004) IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int Immunol 16:249–256
Acknowledgements
We would like to thank Dr. Hermann Rohrer (Max Planck Institute for Brain Research, Frankfurt, Germany) for the DβH-CreER mouse line and Dr. Anton Maximov (Scripps Research Institute, La Jolla, CA, USA) for the Ai9 reporter mouse line.
Funding
This project received support from the National Multiple Sclerosis Society (RG 1501-02646), the Cousins Center for Psychoneuroimmunology, UCLA Semel Institute, and the NIH/NCATS UCLA CTSI (Grant Number UL1TR000124). The latter award helped fund the generation of the PAC1flox/flox DβH-CreER mice.
Microscopy and cryosectioning was performed using instruments made available through the UCLA Intellectual and Developmental Disabilities Research Center (IDDRC) Core. The IDDRC is supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health (5U54HD087101-03) and is an Organized Research Unit supported by the Jane and Terry Semel Institute for Neuroscience and Human Behavior.
Flow cytometry was performed in the UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research Flow Cytometry Core Facility that is supported by National Institutes of Health awards P30 CA016042 and 5P30 AI028697 and by the JCCC, the UCLA AIDS Institute, the David Geffen School of Medicine at UCLA, the UCLA Chancellor’s Office, and the UCLA Vice Chancellor’s Office of Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised: The captured Article Title and corresponding author are incorrect. Full information regarding the corrections made can be found in the correction for this article.
Rights and permissions
About this article
Cite this article
Van, C., Condro, M.C., Lov, K. et al. PACAP/PAC1 Regulation of Inflammation via Catecholaminergic Neurons in a Model of Multiple Sclerosis. J Mol Neurosci 68, 439–451 (2019). https://doi.org/10.1007/s12031-018-1137-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-018-1137-8