Abstract
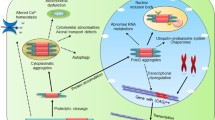
Polyglutamine (poly(Q)) disorders, such as Huntington’s disease (HD) and spinocerebellar ataxias, represent a group of neurological disorders which arise due to an atypically expanded poly(Q) tract in the coding region of the affected gene. Pathogenesis of these disorders inside the cells begins with the assembly of these mutant proteins in the form of insoluble inclusion bodies (IBs), which progressively sequester several vital cellular transcription factors and other essential proteins, and finally leads to neuronal dysfunction and apoptosis. We have shown earlier that targeted upregulation of Drosophila myc (dmyc) dominantly suppresses the poly(Q) toxicity in Drosophila. The present study examines the ability of the human c-myc proto-oncogene and also identifies the specific c-Myc isoform which drives the mitigation of poly(Q)-mediated neurotoxicity, so that it could be further substantiated as a potential drug target. We report for the first time that similar to dmyc, tissue-specific induced expression of human c-myc also suppresses poly(Q)-mediated neurotoxicity by an analogous mechanism. Among the three isoforms of c-Myc, the rescue potential was maximally manifested by the full-length c-Myc2 protein, followed by c-Myc1, but not by c-MycS which lacks the transactivation domain. Our study suggests that strategies focussing on the transactivation domain of c-Myc could be a very useful approach to design novel drug molecules against poly(Q) disorders.





Similar content being viewed by others
References
Benassayag C, Montero L, Colombié N, Gallant P, Cribbs D, Morello D (2005) Human c-Myc isoforms differentially regulate cell growth and apoptosis in Drosophila melanogaster. Mol Cell Biol 25:9897–9909
Bonini NM (1999) A genetic model for human polyglutamine-repeat disease in Drosophila melanogaster. Philos Trans R Soc Lond Ser B Biol Sci 354:1057–1060
Brehme M, Voisine C (2016) Model systems of protein-misfolding diseases reveal chaperone modifiers of proteotoxicity. Dis Model Mech 9:823–838
Chai Y, Koppenhafer SL, Bonini NM, Paulson HL (1999) Analysis of the role of heat shock protein (Hsp) molecular chaperones in polyglutamine diseases. J Neurosci 19:10338–10347
Chan HY, Warrick JM, Andriola I, Merry D, Bonini NM (2002) Genetic modulation of polyglutamine toxicity by protein conjugation pathways in Drosophila. Hum Mol Genet 11:2895–2904
Chen S, Berthelier V, Yang W, Wetzel R (2001) Polyglutamine aggregation behavior in vitro supports a recruitment mechanism of cytotoxicity. J Mol Biol 311:173–182
Claassen GF, Hann SR (1999) Myc-mediated transformation: the repression connection. Oncogene 18:2925–2933
Cole MD, McMahon SB (1999) The Myc oncoprotein: a critical evaluation of transactivation and target gene regulation. Oncogene 18:2916–2924
Evan GI, Lewis GK, Ramsay G, Bishop JM (1985) Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol 5:3610–3616
Everett CM, Wood NW (2004) Trinucleotide repeats and neurodegenerative disease. Brain 127:2385–2405
Ferrer I, Blanco R (2000) N-myc and c-myc expression in Alzheimer disease, Huntington disease and Parkinson disease. Brain Res Mol Brain Res 77:270–276
Hay BA, Wolff T, Rubin GM (1994) Expression of baculovirus P35 prevents cell death in Drosophila. Development 120:2121–2129
Henriksson M, Luscher B (1996) Proteins of the Myc network: essential regulators of cell growth and differentiation. Adv Cancer Res 68:109–182
Ishihara K, Yamagishi N, Saito Y, Adachi H, Kobayashi Y, Sobue G, Ohtsuka K, Hatayama T (2003) Hsp105α suppresses the aggregation of truncated androgen receptor with expanded CAG repeats and cell toxicity. J Biol Chem 278:25143–25150
Jana NR, Tanaka M, Wang G, Nukina N (2000) Polyglutamine length-dependent interaction of Hsp40 and HSp70 family chaperones with truncated N-terminal huntingtin: their role in suppression of aggregation and cellular toxicity. Hum Mol Genet 9:2009–2018
Kobayashi Y, Kume A, Li M, Doyu M, Hata M, Ohtsuka K, Sobue G (2000) Chaperones Hsp70 and Hsp40 suppress aggregate formation and apoptosis in cultured neuronal cells expressing truncated androgen receptor protein with expanded polyglutamine tract. J Biol Chem 275:8772–8778
Lin X, Antalffy B, Kang D, Orr HT, Zoghbi HY (2000) Polyglutamine expansion downregulates specific neuronal genes before pathologic changes in SCA1. Nat Neurosci 3:157–163
Lu B, Vogel H (2009) Drosophila models of neurodegenerative diseases. Annu Rev Pathol 4:315–342
Meyer N, Penn LZ (2008) Reflecting on 25 years of Myc. Nat Rev Cancer 8:976–990
Pereira C, Bessa C, Soares J, Leao M, Saraiva L (2012) Contribution of yeast models to neurodegeneration research. J Biomed Biotechnol. doi:10.1155/2012/941232
Quinn WG, Harris WA, Benzer S (1974) Conditioned behaviour in Drosophila eyes. Proc Natl Acad Sci U S A 71:708–712
Sakamuro D, Prendergast GC (1999) New Myc-interacting proteins: a second Myc network emerges. Oncogene 18:2942–2954
Shao J, Diamond MI (2007) Polyglutamine diseases: emerging concepts in pathogenesis and therapy. Hum Mol Genet 16:R115–R123
Singh MD, Raj K, Sarkar S (2014) Drosophila Myc, a novel modifier suppresses the poly(Q) toxicity by modulating the level of CREB binding protein and histone acetylation. Neurobiol Dis 63:48–61
Steinert JR, Campesan S, Richards P, Kyriacou CP, Forsythe ID, Giorgini F (2012) Rab11 rescues synaptic dysfunction and behavioural deficits in a Drosophila model of Huntington’s disease. Hum Mol Genet 21:2912–2922
Teschendorf D, Link CD (2009) What have worm models told us about the mechanisms of neuronal dysfunction in human neurodegenerative diseases? Mol Neurodegener 4:38
Warrick JM, Chan HY, Gray-Board GL, Chai Y, Paulson HL, Bonini NM (1999) Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat Genet 23:425–428
Weiss KR, Kimura Y, Lee WC, Littleton JT (2012) Huntingtin aggregation kinetics and their pathological role in Drosophila Huntington’s disease model. Genetics 190:581–600
Xiao Q, Claassen G, Shi J, Adachi S, Sedivy J, Hann SR (1998) Transactivation-defective c-MycS retains the ability to regulate proliferation and apoptosis. Genes Dev 12:3803–3808
Acknowledgements
We are thankful to Prof. J. Troy Littleton (MIT, USA) and Prof. Benassayag (Université Paul Sabatier, Toulouse, France) for providing some fly stocks utilised in this study. We also thank Bloomington Stock Center, Indiana, USA, for fly stocks. This work was supported by a research grant (Ref. no. BT/PR4937/MED/30/727/2012) from the Department of Biotechnology (DBT), Government of India, New Delhi, to S.S. KR is supported by the Senior Research Fellowship (SRF) from University Grant Commission (UGC), New Delhi. We also thank Delhi University for the financial support under R&D and DU/DST-PURSE schemes. The financial support to the department under DSTFIST(L2) is thankfully acknowledged. We are grateful to Ms. Nabanita Sarkar and Ms. Soram Idiyasan Chanu for the technical help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raj, K., Sarkar, S. Transactivation Domain of Human c-Myc Is Essential to Alleviate Poly(Q)-Mediated Neurotoxicity in Drosophila Disease Models. J Mol Neurosci 62, 55–66 (2017). https://doi.org/10.1007/s12031-017-0910-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-017-0910-4