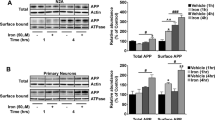
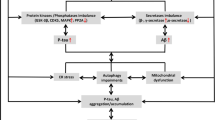
Abstract
An imbalance in metal homeostasis is a prominent feature of Alzheimer’s disease (AD). A wealth of evidence from independent studies over the past two and half decades has found changes to the distribution of brain iron, zinc, and copper in AD patients and animal models of the disease. Early research focused on the association of these metals with amyloid beta (Aβ), particularly extraneuronal Aβ plaque pathology. In contrast, there are numerous studies that have demonstrated a loss of iron-, zinc-, or copper-dependent cellular functions in AD animal and cell models, highlighting the importance of metal homeostasis in maintaining healthy brain function. Characterizing the molecular pathways that are impacted by iron, zinc, or copper will shed light on how these metals affect neuoroprotection, and conversely, neurodegeneration. Of particular interest is the role that the AD-associated presenilins have on protein trafficking and degradation, as metal homeostasis is dependent on the efficient trafficking and recycling of specific metal transporters. This review summarizes what is currently known about presenilin-dependent protein trafficking and the role of presenilin in protein turnover, particularly via the autophagy-lysosomal system.
Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- APP:
-
Amyloid precursor protein
- ER:
-
Endoplasmic reticulum
- PSEN:
-
Presenilin
References
Adlard PA et al. (2010) Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer’s disease? J Neurosci 30(5):1631–1636
Andersson DA et al. (2009) Clioquinol and pyrithione activate TRPA1 by increasing intracellular Zn2+. Proc Natl Acad Sci U S A 106(20):8374–8379
Annaert WG et al. (2001) Interaction with telencephalin and the amyloid precursor protein predicts a ring structure for presenilins. Neuron 32(4):579–589
Avrahami L et al. (2013) Inhibition of glycogen synthase kinase-3 ameliorates beta-amyloid pathology and restores lysosomal acidification and mammalian target of rapamycin activity in the Alzheimer disease mouse model: in vivo and in vitro studies. J Biol Chem 288(2):1295–1306
Bellingham SA et al. (2004) Gene knockout of amyloid precursor protein and amyloid precursor-like protein-2 increases cellular copper levels in primary mouse cortical neurons and embryonic fibroblasts. J Neurochem 91(2):423–428
Beranger F et al. (2002) Stimulation of PrP(C) retrograde transport toward the endoplasmic reticulum increases accumulation of PrP(Sc) in prion-infected cells. J Biol Chem 277(41):38972–38977
Boonen RA, van Tijn P, Zivkovic D (2009) Wnt signaling in Alzheimer’s disease: up or down, that is the question. Ageing Res Rev 8(2):71–82
Bush AI The Metal Theory of Alzheimer’s Disease. J Alzheimers Dis, 2012
Cai D et al. (2003) Presenilin-1 regulates intracellular trafficking and cell surface delivery of beta-amyloid precursor protein. J Biol Chem 278(5):3446–3454
Cai D et al. (2006) Phospholipase D1 corrects impaired betaAPP trafficking and neurite outgrowth in familial Alzheimer’s disease-linked presenilin-1 mutant neurons. Proc Natl Acad Sci U S A 103(6):1936–1940
Camello C et al. (2002) Calcium leak from intracellular stores--the enigma of calcium signalling. Cell Calcium 32(5–6):355–361
Cataldo AM et al. (1996) Colocalization of lysosomal hydrolase and beta-amyloid in diffuse plaques of the cerebellum and striatum in Alzheimer’s disease and Down’s syndrome. J Neuropathol Exp Neurol 55(6):704–715
Cataldo AM et al. (2000) Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am J Pathol 157(1):277–286
Cataldo AM et al. (2004) Presenilin mutations in familial Alzheimer disease and transgenic mouse models accelerate neuronal lysosomal pathology. J Neuropathol Exp Neurol 63(8):821–830
Chen Q, Schubert D (2002) Presenilin-interacting proteins. Expert Rev Mol Med 4(19):1–18
Chen F et al. (2000) Carboxyl-terminal fragments of Alzheimer beta-amyloid precursor protein accumulate in restricted and unpredicted intracellular compartments in presenilin 1-deficient cells. J Biol Chem 275(47):36794–36802
Cirrito JR et al. (2008) Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron 58(1):42–51
Coen, K., W. Annaert, Presenilins: how much more than gamma-secretase?! Biochem Soc Trans, 2010. 38(6): 1474–1478.
Coen K et al. (2012) Lysosomal calcium homeostasis defects, not proton pump defects, cause endo-lysosomal dysfunction in PSEN-deficient cells. J Cell Biol 198(1):23–35
Dobrowolski R et al. (2012) Presenilin deficiency or lysosomal inhibition enhances Wnt signaling through relocalization of GSK3 to the late-endosomal compartment. Cell Rep 2(5):1316–1328
Duce JA et al. (2010) Iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in Alzheimer’s disease. Cell 142(6):857–867
Esselens C et al. (2004) Presenilin 1 mediates the turnover of telencephalin in hippocampal neurons via an autophagic degradative pathway. J Cell Biol 166(7):1041–1054
Frederickson CJ, Koh JY, Bush AI (2005) The neurobiology of zinc in health and disease. Nat Rev Neurosci 6(6):449–462
Gandy S et al. (2007) Alzheimer’s presenilin 1 modulates sorting of APP and its carboxyl-terminal fragments in cerebral neurons in vivo. J Neurochem 102(3):619–626
Gowrishankar K, Zeidler MG, Vincenz C (2004) Release of a membrane-bound death domain by gamma-secretase processing of the p75NTR homolog NRADD. J Cell Sci 117(Pt 18):4099–4111
Greenough MA, Camakaris J, Bush AI (2013) Metal dyshomeostasis and oxidative stress in Alzheimer’s disease. Neurochem Int 62(5):540–555
Greenough MA et al. (2011) Presenilins promote the cellular uptake of copper and zinc and maintain copper chaperone of SOD1-dependent copper/zinc superoxide dismutase activity. J Biol Chem 286(11):9776–9786
Gu Q, Lin RL (2010a) Heavy metals zinc, cadmium, and copper stimulate pulmonary sensory neurons via direct activation of TRPA1. J Appl Physiol (1985) 108(4):891–897
Gu Q, Lin RL (2010b) Heavy metals zinc, cadmium, and copper stimulate pulmonary sensory neurons via direct activation of TRPA1. J Appl Physiol 108(4):891–897
Hung YH et al. (2009) Paradoxical condensation of copper with elevated beta-amyloid in lipid rafts under cellular copper deficiency conditions: implications for Alzheimer disease. J Biol Chem 284(33):21899–21907
Jin LW et al. (2004) Intracellular accumulation of amyloidogenic fragments of amyloid-beta precursor protein in neurons with Niemann-Pick type C defects is associated with endosomal abnormalities. Am J Pathol 164(3):975–985
Kaether C et al. (2002) Presenilin-1 affects trafficking and processing of betaAPP and is targeted in a complex with nicastrin to the plasma membrane. J Cell Biol 158(3):551–561
Kametani F et al. (2004) Mutant presenilin (A260V) affects Rab8 in PC12D cell. Neurochem Int 44(5):313–320
Kang DE et al. (2005) Presenilins mediate phosphatidylinositol 3-kinase/AKT and ERK activation via select signaling receptors. Selectivity of PS2 in platelet-derived growth factor signaling. J Biol Chem 280(36):31537–31547
Kepp KP Alzheimer’s disease due to loss of function: a new synthesis of the available data. Prog Neurobiol, 2016
Koo EH, Squazzo SL (1994) Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem 269(26):17386–17389
Koo EH et al. (1996) Trafficking of cell-surface amyloid beta-protein precursor. I. Secretion, endocytosis and recycling as detected by labeled monoclonal antibody. J Cell Sci 109(Pt 5):991–998
LaFerla FM (2002) Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat Rev Neurosci 3(11):862–872
Lee JH et al. (2010) Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 141(7):1146–1158
Lee JH et al. (2015) Presenilin 1 maintains lysosomal Ca(2+) homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification. Cell Rep 12(9):1430–1444
Leem JY et al. (2002) A role for presenilin 1 in regulating the delivery of amyloid precursor protein to the cell surface. Neurobiol Dis 11(1):64–82
Li Y et al. (2001) Induction of mossy fiber --> Ca3 long-term potentiation requires translocation of synaptically released Zn2+. J Neurosci 21(20):8015–8025
Li D et al. (2006) Mutations of presenilin genes in dilated cardiomyopathy and heart failure. Am J Hum Genet 79(6):1030–1039
Li H et al. (2011a) Polymorphisms of presenilin-1 gene associate with dilated cardiomyopathy susceptibility. Mol Cell Biochem 358(1–2):31–36
Li A et al. (2011b) Changes in the expression of the Alzheimer’s disease-associated presenilin gene in drosophila heart leads to cardiac dysfunction. Curr Alzheimer Res 8(3):313–322
Lingwood D, Simons K (2010) Lipid rafts as a membrane-organizing principle. Science 327(5961):46–50
Lloyd-Evans E et al. (2008) Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med 14(11):1247–1255
Lothian A et al. (2013) Metalloproteomics: principles, challenges and applications to neurodegeneration. Front Aging Neurosci 5:35
Lu YM et al. (2000) Endogenous Zn(2+) is required for the induction of long-term potentiation at rat hippocampal mossy fiber-CA3 synapses. Synapse 38(2):187–197
Matias CM et al. (2010) Validation of TPEN as a zinc chelator in fluorescence probing of calcium in cells with the indicator Fura-2. J Fluoresc 20(1):377–380
McCarthy JV (2005) Involvement of presenilins in cell-survival signalling pathways. Biochem Soc Trans 33(Pt 4):568–572
McConlogue L et al. (1996) Differential effects of a Rab6 mutant on secretory versus amyloidogenic processing of Alzheimer’s beta-amyloid precursor protein. J Biol Chem 271(3):1343–1348
Mortimore GE, Poso AR (1987) Intracellular protein catabolism and its control during nutrient deprivation and supply. Annu Rev Nutr 7:539–564
Mukhopadhyay I et al. (2011) Expression of functional TRPA1 receptor on human lung fibroblast and epithelial cells. J Recept Signal Transduct Res 31(5):350–358
Murayama M et al. (1998) Direct association of presenilin-1 with beta-catenin. FEBS Lett 433(1–2):73–77
Naruse S et al. (1998) Effects of PS1 deficiency on membrane protein trafficking in neurons. Neuron 21(5):1213–1221
Neely KM, Green KN, LaFerla FM (2011) Presenilin is necessary for efficient proteolysis through the autophagy-lysosome system in a gamma-secretase-independent manner. J Neurosci 31(8):2781–2791
Nelson O et al. (2007) Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J Clin Invest 117(5):1230–1239
Nelson O et al. (2011) Mutagenesis mapping of the presenilin 1 calcium leak conductance pore. J Biol Chem 286(25):22339–22347
Norgate M et al. (2006) Essential roles in development and pigmentation for the Drosophila copper transporter DmATP7. Mol Biol Cell 17(1):475–484
Norgate M et al. (2010) Syntaxin 5 is required for copper homeostasis in Drosophila and mammals. PLoS One 5(12):e14303
Nornes S et al. (2008) Interference with splicing of presenilin transcripts has potent dominant negative effects on presenilin activity. Hum Mol Genet 17(3):402–412
Ohta K et al. (2010) Autophagy impairment stimulates PS1 expression and gamma-secretase activity. Autophagy 6(3):345–352
Pan E et al. (2011) Vesicular zinc promotes presynaptic and inhibits postsynaptic long-term potentiation of mossy fiber-CA3 synapse. Neuron 71(6):1116–1126
Perez RG et al. (1999) Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Abeta42. J Biol Chem 274(27):18851–18856
Pigino G et al. (2001) Presenilin-1 mutations reduce cytoskeletal association, deregulate neurite growth, and potentiate neuronal dystrophy and tau phosphorylation. J Neurosci 21(3):834–842
Rechards M et al. (2006) Presenilin-1-mediated retention of APP derivatives in early biosynthetic compartments. Traffic 7(3):354–364
Repetto E et al. (2007) Presenilin 1 regulates epidermal growth factor receptor turnover and signaling in the endosomal-lysosomal pathway. J Biol Chem 282(43):31504–31516
Ring S et al. (2007) The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J Neurosci 27(29):7817–7826
Roe MW, Lemasters JJ, Herman B (1990) Assessment of Fura-2 for measurements of cytosolic free calcium. Cell Calcium 11(2–3):63–73
Saftig P, Klumperman J (2009) Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol 10(9):623–635
Sandhoff K, Andreae U, Jatzkewitz H (1968) Deficient hexosaminidase activity in an exceptional case of Tay-Sachs disease with additional storage of kidney globoside in visceral organs. Pathol Eur 3(2):278–285
Sato C et al. (2006) Structure of the catalytic pore of gamma-secretase probed by the accessibility of substituted cysteines. J Neurosci 26(46):12081–12088
Saura CA et al. (2004) Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron 42(1):23–36
Scheper W et al. (2000) Alzheimer’s presenilin 1 is a putative membrane receptor for rab GDP dissociation inhibitor. Hum Mol Genet 9(2):303–310
Scheper W, Zwart R, Baas F (2004) Rab6 membrane association is dependent of presenilin 1 and cellular phosphorylation events. Brain Res Mol Brain Res 122(1):17–23
Shen J, Kelleher RJ III (2007) The presenilin hypothesis of Alzheimer’s disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci U S A 104(2):403–409
Shepherd CE et al. (2004) Positional effects of presenilin-1 mutations on tau phosphorylation in cortical plaques. Neurobiol Dis 15(1):115–119
Shilling D et al. (2012) Lack of evidence for presenilins as endoplasmic reticulum Ca2+ leak channels. J Biol Chem 287(14):10933–10944
Small SA, Gandy S (2006) Sorting through the cell biology of Alzheimer’s disease: intracellular pathways to pathogenesis. Neuron 52(1):15–31
Smith IF, Green KN, LaFerla FM (2005) Calcium dysregulation in Alzheimer’s disease: recent advances gained from genetically modified animals. Cell Calcium 38(3–4):427–437
Snitsarev VA, McNulty TJ, Taylor CW (1996) Endogenous heavy metal ions perturb fura-2 measurements of basal and hormone-evoked Ca2+ signals. Biophys J 71(2):1048–1056
Southon A et al. (2013) Presenilin promotes dietary copper uptake. PLoS One 8(5):e62811
Stutzmann GE (2005) Calcium dysregulation, IP3 signaling, and Alzheimer’s disease. Neuroscientist 11(2):110–115
Suga K et al. (2004) Syntaxin 5 interacts with presenilin holoproteins, but not with their N- or C-terminal fragments, and affects beta-amyloid peptide production. Biochem J 381(Pt 3):619–628
Suga K et al. (2005) Syntaxin 5 interacts specifically with presenilin holoproteins and affects processing of betaAPP in neuronal cells. J Neurochem 94(2):425–439
Suga K et al. (2009) The Syntaxin 5 isoforms Syx5 and Syx5L have distinct effects on the processing of {beta}-amyloid precursor protein. J Biochem 146(6):905–915
Thinakaran G, Koo EH (2008) Amyloid precursor protein trafficking, processing, and function. J Biol Chem 283(44):29615–29619
Tolia A, Chavez-Gutierrez L, De Strooper B (2006) Contribution of presenilin transmembrane domains 6 and 7 to a water-containing cavity in the gamma-secretase complex. J Biol Chem 281(37):27633–27642
Tu H et al. (2006) Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell 126(5):981–993
Uemura K et al. (2003) Presenilin 1 is involved in maturation and trafficking of N-cadherin to the plasma membrane. J Neurosci Res 74(2):184–191
Venezia V et al. (2007) Amyloid precursor protein and presenilin involvement in cell signaling. Neurodegener Dis 4(2–3):101–111
Wang H et al. (2004) Presenilins and gamma-secretase inhibitors affect intracellular trafficking and cell surface localization of the gamma-secretase complex components. J Biol Chem 279(39):40560–40566
Wang HQ et al. (2005) Interaction of presenilins with FKBP38 promotes apoptosis by reducing mitochondrial Bcl-2. Hum Mol Genet 14(13):1889–1902
Wang R et al. (2006) Regulation of tyrosinase trafficking and processing by presenilins: partial loss of function by familial Alzheimer’s disease mutation. Proc Natl Acad Sci U S A 103(2):353–358
Wilson CA et al. (2004) Degradative organelles containing mislocalized alpha-and beta-synuclein proliferate in presenilin-1 null neurons. J Cell Biol 165(3):335–346
Wolfe DM et al. (2013) Autophagy failure in Alzheimer’s disease and the role of defective lysosomal acidification. Eur J Neurosci 37(12):1949–1961
Wong BX et al. (2014) beta-Amyloid precursor protein does not possess ferroxidase activity but does stabilize the cell surface ferrous iron exporter ferroportin. PLoS One 9(12):e114174
Wrigley JD et al. (2005) Functional overexpression of gamma-secretase reveals protease-independent trafficking functions and a critical role of lipids for protease activity. J Biol Chem 280(13):12523–12535
Yamazaki T, Koo EH, Selkoe DJ (1996) Trafficking of cell-surface amyloid beta-protein precursor. II. Endocytosis, recycling and lysosomal targeting detected by immunolocalization. J Cell Sci 109(Pt 5):999–1008
Yu JT, Chang RC, Tan L (2009) Calcium dysregulation in Alzheimer’s disease: from mechanisms to therapeutic opportunities. Prog Neurobiol 89(3):240–255
Zhang Z et al. (1998) Destabilization of beta-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature 395(6703):698–702
Zhang M et al. (2006) Presenilin/gamma-secretase activity regulates protein clearance from the endocytic recycling compartment. FASEB J 20(8):1176–1178
Zhang X et al. (2012) A role for presenilins in autophagy revisited: normal acidification of lysosomes in cells lacking PSEN1 and PSEN2. J Neurosci 32(25):8633–8648
Zou K et al. (2008) Novel role of presenilins in maturation and transport of integrin beta 1. Biochemistry 47(11):3370–3378
Acknowledgments
The Florey Institute of Neuroscience and Mental Health acknowledges the strong support from the Victorian Government and in particular the funding from the Operational Infrastructure Support Grant. This work was supported by the Australian National Health and Medical Research Council (NHMRC) and the Australian Research Council (ARC). MAG is the recipient of a NHMRC Dementia Research Fellowship. I would personally like to thank Professor Ashley Bush for his continued guidance and support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Greenough, M. The Role of Presenilin in Protein Trafficking and Degradation—Implications for Metal Homeostasis. J Mol Neurosci 60, 289–297 (2016). https://doi.org/10.1007/s12031-016-0826-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-016-0826-4