Abstract
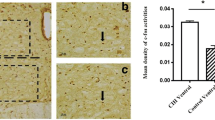
The aim of this study is to investigate whether systemic 5-hydroxytryptamine (5-HT) can promote long-lasting form of respiratory plasticity in vivo via 5-HT2AR-activated protein kinase C (PKC) mechanism. The frequency and peak amplitude of hypoglossal nerve discharges in anesthetized rats were compared before and after intravenous injections of different treatments, including saline, 5-HT, ketanserin tartrate, or staurosporine. The administration of 5-HT at a systemic bolus imposed an initial ephemeral inhibition subsequently followed by striking facilitation, which demonstrates a biphasic manner of hypoglossal nerve output in anesthetized adult rats. The facilitatory stage conformed to the reinforced hypoglossal activity that lasted for more than 60 min after drug administration. The 5-HT evoked biphasic manner of hypoglossal output and hypoglossal nerve activity LTF (hLTF) were 5-HT2A receptor-dependent and coupled to PKC activation. The initial inhibition of hypoglossal activity was associated with nodose ganglion, and the subsequent facilitation was associated with carotid body. The reactive oxygen species (ROS) formation was triggered in the systemic 5-HT2-dependent hLTF model in vivo. The expressions of immunofluorescent histochemistry provide morphological evidence of a 5-HT/5-HT2A receptor coupled to PKC mechanism. In conclusion, systemic 5-HT challenge contributes to long-lasting form of respiratory plasticity and to elicit hLTF or elevated hLTF in animals, which with decreased or even with inhibited peripheral inhibitory activations. The effect of systemic 5-HT was regulated by a 5-HT2AR-activated PKC mechanism.







Similar content being viewed by others
References
Bach KB, Mitchell GS (1996) Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104(2-3):251–260
Baker-Herman TL, Strey KA (2011) Similarities and differences in mechanisms of phrenic and hypoglossal motor facilitation. Respir Physiol Neurobiol 179(1):48–56
Batsikadze G, Paulus W, Kuo MF, Nitsche MA (2013) Effect of serotonin on paired associative stimulation-induced plasticity in the human motor cortex. Neuropsychopharmacology 38(11):2260–2267
Bavis RW, Mitchell GS (2002) Plasticity in respiratory motor control selected contribution: intermittent hypoxia induces phrenic long-term facilitation in carotid body-denervated rats. J Appl Physiol 94(1):399–409
Bhattacharyya S, Puri S, Miledi R, Panicker MM (2002) Internalization and recycling of 5-HT2A receptors activated by serotonin and protein kinase C-mediated mechanisms. Proc Natl Acad Sci U S A 99(22):14470–14475
Bocchiaro CM, Feldman JL (2004) Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci U S A 101(12):4292–4295
Bonham AC (1995) Neurotransmitters in the CNS control of breathing. Respir Physiol 101(3):219–230
Carley DW, Radulovacki M (2008) Pharmacology of vagal afferent influences on disordered breathing during sleep. Respir Physiol Neurobiol 164(1-2):197–203
Coleridge JCG, Coleridge HM (1984) Afferent vagal C-fiber innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99(1):1–110
Feldman JL, Mitchell GS, Nattie EE (2003) Breathing: rhythmicity, plasticity, chemo- sensitivity. Annu Rev Neurosci 26(1):239–266
Fuller DD, Bach KB, Kinkead BR, Mitchell GS (2000) Long-term facilitation of phrenic motor output. Respir Physiol 121(2):135–146
Fuller DD, Zabka AG, Baker TL, Mitchell GS (2001) Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol 90(8):2001–2006
Hickner S, Hussain N, Angoa-Perez M, Francescutti DM, Kuhn DM, Mateika JH (2004) Ventilatory long-term facilitation is evident after initial and repeated exposure to intermittent hypoxia in mice genetically depleted of brain serotonin. J Appl Physiol 116(3):240–250
Hilaire G, Bou C, Monteau R (1997) Serotonergic modulation of central respiratory activity in the neonatal mouse: an in vitro study. Eur J Pharmacol 329(2–3):115–120
Hilaire G, Voituron N, Menuet C, Ichiyama RM, Subramanian HH, Dutschmann M (2010) The role of serotonin in respiratory function and dysfunction. Respir Physiol Neurobiol 174(1):76–88
Hoffman MS, Mitchell GS (2013) Spinal 5-HT7 receptors and protein kinase A constrain intermittent hypoxia-induced phrenic long-term facilitation. Neuroscience 250(1):632–643
Hu JY, Glickman L, Wu F, Schacher S (2004) Serotonin regulates the secretion and autocrine action of a neuropeptide to activate MAPK required for long-term facilitation in Aplysia. Neuron 43(3):373–385
Kinney HC (2005) Abnormalities of the brainstem serotonergic system in the sudden infant death syndrome: a review. Pediatr Dev Pathol 8(5):507–524
Kobayashi S, Fujito Y, Matsuyama K, Aoki M (2010) Raphe modulation of the pre-Bötzinger complex respiratory bursts in in vitro medullary half-slice preparations of neonatal mice. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 196(8):519–528
Kopczyńska B, Szereda-Przestaszewska M (2004) 5HT2 and 5HT3 receptors’ contribution to modeling of post-serotonin respiratory pattern in cats. Life Sci 75(19):2281–2290
Lindsay AD, Feldman JL (1993) Modulation of respiratory activity of neonatal rat phrenic motoneurons by serotonin. J Physiol 461(1):213–233
Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Mitchell GS (2001) Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci 21(14):5381–5388
MacFarlane PM, Wilkerson JER, Lovett-Barr MR, Mitchell GS (2008) Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir Physiol Neurobiol 164:263–271
MacFarlane PM, Vinit S, Mitchell GS (2014) Spinal nNOS regulates phrenic motor facilitation by a 5-HT2B receptor- and NADPH oxidase-dependent mechanism. Neuroscience 269(1):67–78
Mahamed S, Mitchell GS (2007) Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol 92:27–37
Mahamed S, Mitchell GS (2008) Simulated apnoeas induce serotonin-dependent respiratory long- term facilitation in rats. J Physiol 586(8):2171–2181
Millhorn DE, Eldridge FL, Waldrop TG (1980) Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol 41(1):87–103
Mitchell GS, Johnson SM (2003) Neuroplasticity in respiratory motor control. J Appl Physiol 94(1):358–374
Mitchell GS, Baker TL, Naada SA (2001) Invited review: intermittent hypoxia and respiratory plasticity. J Appl Physiol 90(6):2466–2475
Morin D, Hennequin S, Monteau R, Hilaire G (1990) Serotonergic influences on central respiratory activity: an in vitro study in the newborn rat. Brain Res 535(2):281–287
Morin D, Monteau R, Hilaire G (1992) Compared effects of serotonin on cervical and hypoglossal inspiratory activities: an in vitro study. J Physiol Lond 451(1):605–629
Neverova NV, Saywell SA, Nashold LJ, Mitchell GS, Feldman JL (2007) Episodic stimulation of alpha1- adrenoreceptors induces protein kinase C-dependent persistent changes in motoneuronal excitability. J Neurosci 27(16):4435–4442
Onimaru H, Shamoto A, Homma I (1998) Modulation of respiratory rhythm by 5-HT in the brainstem-spinal cord preparation from newborn rat. Pflugers Arch 435(4):485–494
Peng YJ, Yuan G, Jacono FJ, Kumar GK, Prabhakar NR (2006) 5-HT evokes sensory long-term facilitation of rodent carotid body via activation of NADPH oxidase. J Physiol 576(Pt1):289–295
Richter DW, Manzke T, Wilken B, Ponimaskin E (2003) Serotonin receptors: guardians of stable breathing. Trends Mol Med 9(12):542–548
Schwarzacher SW, Pestean A, Günther S, Ballanyi K (2002) Serotonergic modulation of respiratory motoneurons and interneurons in brainstem slices of perinatal rats. Neuroscience 115(4):1247–1259
Valic M, Pecotic R, Pavlinac I, Valic Z, Peros K, Dogas Z (2010) Microinjection of methysergide into the raphe nucleus attenuated phrenic long-term facilitation in rats. Exp Brain Res 202(3):583–589
Wilkerson JER, Satriotomo I, Baker-Herman TL, Watters JJ, Mitchell GS (2008) Okadaic acid-sensitive protein phosphatases constrain phrenic long-term facilitation after sustained hypoxia. J Neurosci 28(11):2949–2958
Xing T, Fong AY, Bautista TG, Pilowsky PM (2013) Acute intermittent hypoxia induced neural plasticity in respiratory motor control. Clin Exp Pharmacol Physiol 40(9):602–609
Yoshioka M, Ikeda T, Abe M, Togashi H, Minami M, Saito H (1992) Pharmacological characterization of 5-hydroxytryptamine-induced excitation of afferent cervical vagus nerve in anaesthetized rats. Br J Pharmacol 106(3):544–549
Zhao JJ, Wen FJ, Wang N, Fan H, Wan ZH, Hu K (2012) Construction of an experimental hypoglossal nerve signal record platform in rat model of chronic intermittent hypoxia in vivo. Med J Wuhan Univ 33(3):350–354
Acknowledgments
This work was supported by the National Natural Science Foundation of China under grants No. 81070065 and No. 81370181.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Observation of the effects of Sarpogrelate, Trazodone and Nefazodone on 5-HT-induced hypoglossal activity (amplitudes at 60 min post-injection). A. Effects of Sarpogrelate. B. Effects of Trazodone. C. Effects of Nefazodone. P < 0.01 represents the Amplitude compared to the 5-HT group. (PPT 123 kb)
Supplementary Figure 2
Observation of the effects of Calphostin C, Ro31-8220 and Scutllarein on 5-HT-induced hypoglossal activity (amplitudes at 60 min post-injection). A. Effects of Sarpogrelate. B. Effects of Ro31-8220. C. Effects of Scutllarein. P < 0.01 represents the Amplitude compared to the 5-HT group. (PPT 125 kb)
Rights and permissions
About this article
Cite this article
Tu, X., Zuo, J., Hu, K. et al. Effect of Systemic Application of 5-Hydroxytryptamine on Hypoglossal Nerve Discharge in Anesthetized Rats. J Mol Neurosci 57, 435–445 (2015). https://doi.org/10.1007/s12031-015-0590-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-015-0590-x