Abstract
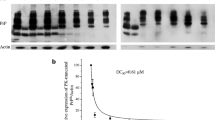
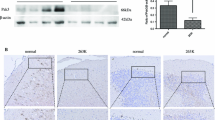
Prion diseases are composed of a group of fatal neurodegenerative disorders resulting from misfolding of cellular prion (PrPC) into scrapie prion (PrPSc). Sirt1, a class III histone deacetylase, has been reported to protect neuronal cells against PrP (106–126)-induced cell death. To address the potential role of Sirt1 during prion infection, the levels and enzyme activities of Sirt1 in the brains of scrapie-infected rodents, including hamsters infected with strain 263K, mice infected with strains 139A and ME7, and in prion infected SMB-S15 cells, were analyzed. Western blots revealed that endogenous Sirt1 levels were significantly decreased in all tested scrapie-infected models. Dynamic assays of brain Sirt1 levels in 263K-infected hamsters during incubation period showed a time-dependent decrease. The acetylating forms of Sirt1 target proteins, P53, PGC-1, and STAT3, markedly increased both in the brains of scrapie-infected rodents and in SMB-S15 cells, representing decreased Sirt1 activity. Immunofluorescent assays illustrated that Sirt1 predominately localized in cytosol of SMB-S15 cells but clearly distributed in nucleus of its normal partner cell line, SMB-PS. Moreover, accompanying with increase of Sirt1 level and decrease of acetyl-P53 level, treatments with Sirt1 activators SRT1720 and resveratrol in SMB-S15 cells significantly reduced PrPSc; at the same time, the cellular distribution of PrP proteins became normal, and the cell proliferating state was slightly improved. These data indicate that prion infection notably attenuates the Sirt1 activity in host cells. Sensitivity of the PrPSc to Sirt1 activators highlights a potential role of Sirt1 in prion therapeutics.





Similar content being viewed by others
References
Aguzzi A (2006) Prion diseases of humans and farm animals: epidemiology, genetics, and pathogenesis. J Neurochem 97:1726–1739
Albani D, Polito L, Signorini A, Forloni G (2010) Neuroprotective properties of resveratrol in different neurodegenerative disorders. Biofactors 36:370–376
Bizat N, Peyrin JM, Haik S et al (2010) Neuron dysfunction is induced by prion protein with an insertional mutation via a Fyn kinase and reversed by sirtuin activation in Caenorhabditis elegans. J Neurosci 30:5394–5403
Chen D, Steele AD, Hutter G et al (2008) The role of calorie restriction and SIRT1 in prion-mediated neurodegeneration. Exp Gerontol 43:1086–1093
Donmez G (2013) Sirtuins as possible targets in neurodegenerative diseases. Curr Drug Targets 14:644–647
Donmez G, Outeiro TF (2013) SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med 5:344–352
Donmez G, Wang D, Cohen DE, Guarente L (2010) SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell 142:320–332
Donmez G, Arun A, Chung CY, McLean PJ, Lindquist S, Guarente L (2012) SIRT1 protects against alpha-synuclein aggregation by activating molecular chaperones. J Neurosci 32:124–132
Duan W (2013) Sirtuins: from metabolic regulation to brain aging. Front Aging Neurosci 5:36
Haig DA, Clarke MC (1971) Multiplication of the scrapie agent. Nature 234:106–107
Ho DJ, Calingasan NY, Wille E, Dumont M, Beal MF (2010) Resveratrol protects against peripheral deficits in a mouse model of Huntington’s disease. Exp Neurol 225:74–84
Jeong JK, Moon MH, Lee YJ, Seol JW, Park SY (2013) Autophagy induced by the class III histone deacetylase Sirt1 prevents prion peptide neurotoxicity. Neurobiol Aging 34:146–156
Khan RS, Fonseca-Kelly Z, Callinan C, Zuo L, Sachdeva MM, Shindler KS (2012) SIRT1 activating compounds reduce oxidative stress and prevent cell death in neuronal cells. Front Cell Neurosci 6:63
Kretzschmar HA (1999) Molecular pathogenesis of prion diseases. Eur Arch Psychiatry Clin Neurosci 249(Suppl 3):56–63
Luo J, Nikolaev AY, Imai S et al (2001) Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107:137–148
Mattson MP, Magnus T (2006) Ageing and neuronal vulnerability. Nat Rev Neurosci 7:278–294
Milne JC, Lambert PD, Schenk S et al (2007) Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450:712–716
Min SW, Cho SH, Zhou Y et al (2010) Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron 67:953–966
Min SW, Sohn PD, Cho SH, Swanson RA, Gan L (2013) Sirtuins in neurodegenerative diseases: an update on potential mechanisms. Front Aging Neurosci 5:53
Minor RK, Baur JA, Gomes AP et al (2011) SRT1720 improves survival and healthspan of obese mice. Sci Rep 1:70
Mitchell SJ, Martin-Montalvo A, Mercken EM et al (2014) The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep 6:836–843
Nayagam VM, Wang X, Tan YC et al (2006) SIRT1 modulating compounds from high-throughput screening as anti-inflammatory and insulin-sensitizing agents. J Biomol Screen 11:959–967
Nemoto S, Fergusson MM, Finkel T (2005) SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha}. J Biol Chem 280:16456–16460
Pallas M, Porquet D, Vicente A, Sanfeliu C (2013) Resveratrol: new avenues for a natural compound in neuroprotection. Curr Pharm Des 19:6726–6731
Prusiner SB (1998) Prions. Proc Natl Acad Sci U S A 95:13363–13383
Qin W, Yang T, Ho L et al (2006) Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem 281:21745–21754
Rahman S, Islam R (2011) Mammalian Sirt1: insights on its biological functions. Cell Commun Signal 9:11
Ramadori G, Lee CE, Bookout AL et al (2008) Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci 28:9989–9996
Seo JS, Moon MH, Jeong JK et al (2012) SIRT1, a histone deacetylase, regulates prion protein-induced neuronal cell death. Neurobiol Aging 33:1110–1120
Shi Q, Zhang BY, Gao C et al (2012) Mouse-adapted scrapie strains 139A and ME7 overcome species barrier to induce experimental scrapie in hamsters and changed their pathogenic features. Virol J 9:63
Sorolla MA, Nierga C, Rodriguez-Colman MJ et al (2011) Sir2 is induced by oxidative stress in a yeast model of Huntington disease and its activation reduces protein aggregation. Arch Biochem Biophys 510:27–34
Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y (2007) Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem 282:6823–6832
Yeung F, Hoberg JE, Ramsey CS et al (2004) Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23:2369–2380
Acknowledgments
This work was supported by the Chinese National Natural Science Foundation Grants (81100980, 81301429), SKLID Development Grant (2013SKLID402, 2012SKLID102), and China Mega-Project for Infectious Disease (2011ZX10004-101, 2012ZX10004215).
Conflict of Interest
The authors report no conflicts of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Zhang, J., Shi, Q. et al. Scrapie Infection in Experimental Rodents and SMB-S15 Cells Decreased the Brain Endogenous Levels and Activities of Sirt1. J Mol Neurosci 55, 1022–1030 (2015). https://doi.org/10.1007/s12031-014-0459-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-014-0459-4