Abstract
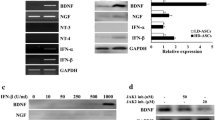
Autophagy plays different roles in the growth and development process of different cells. The role of autophagy in the differentiation process of adult adipose-derived stromal cells (ADSCs) into astrocytes is unclear. We researched the role of autophagy in the induction process by adding autophagy agonist rapamycin, which was not added in the control group. Immunocytochemistry showed that the expression of glial fibrillary acidic protein (GFAP) was increased gradually with the extending reaction time and had reached the peak on the 14th day. Typical autophagy ultrastructure, including autophagic bodies and self-macrophage lysosomal, was shown under transmission electron microscopy (TEM) when cells were induced for 14 days. By methyl thiazolyl tetrazolium (MTT) assay, we found that the number of living cells was reduced gradually, and early apoptosis rate was increased by flow cytometry. We observed that the differentiation ratio, the number of living cells, and the positive expression rates of GFAP in the rapamycin group were higher than those in the control group when ADSCs were induced for 48 h and 7 days (P < 0.01); however, the rates of early apoptosis were lower than those in the control group. The positive rate of microtubule-associated protein light chain 3 (LC3) in the rapamycin group had been up to 88.87 % on the 7th day (P < 0.01), but not obvious with extending time. After 14 days of induction, the optical density (OD) value of surviving cells was declined, and early apoptosis rate was increased gradually. The results showed that adding autophagy agonist to the inducers may enhance intensity of autophagy, shorten the induction time, and improve the efficiency of differentiation.





Similar content being viewed by others
References
Cai YN, Yuan XD, Ou Y, Lu YH (2011) Apoptosis during β-mercaptoethanol-induced differentiation of adult adipose-derived stromal cells into neurons. Neural Regen Res 6(10):750–755
Carloni S, Girelli S, Scopa C, Buonocore G, Longini M, Balduini W (2010) Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia-ischemia. Autophagy 6(3):366–377
Chu CT (2008) Eaten alive: autophagy and neuronal cell death after hypoxia-ischemia. Am J Pathol 172(2):284–287
Dadakhujaev S, Noh HS, Jung EJ et al (2010) Autophagy protects the rotenone-induced cell death in alpha-synuclein overexpressing SH-SY5Y cells. Neuroscience letters 472(1):47–52
Eskelinen EL, Saftig P (2009) Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochim Biophys Acta 1793(4):664–673
Eskelinen EL, Reggiori F, Baba M, Kovacs AL, Seglen PO (2011) Seeing is believing: the impact of electron microscopy on autophagy research. Autophagy 7(9):935–956
Fang YQ, Liu L (2011) Review in neuronal autophagy. Chinese Archives of Traditional Chinese Medicine 29(3):453–455
Ginet V, Puyal J, Clarke PG, Truttmann AC (2009) Enhancement of autophagic flux after neonatal cerebral hypoxia-ischemia and its region-specific relationship to apoptotic mechanisms. Am J Pathol 175(5):1962–1974
Gomes FC, Paulin D, Moura NV (1999) Glial fibrillary acidic protein (GFAP): modulation by growth factors and its implication in astrocyte differentiation. Braz J Med Biol Res 32(5):619–631
Holt SV, Wyspianska B, Randall KJ, James D, Foster JR, Wilkinson RW (2011) The development of an immunohistochemical method to detect the autophagy-associated protein LC3II in human tumour xenografts. Toxicol Pathol 39(3):516–523
Jiang H, Cheng D, Liu W, Peng J, Feng J (2010) Protein kinase C inhibits autophagy and phosphorylates LC3. Biochem Biophys Res Commun 395(4):471–476
Liu H, Yuan XD, Ye CQ, Ou Y, Cai YN (2010) Adult adipose-derived stem cells differentiation into astrocyte cells morphology and ultrastructure in vitro. Chin J Behav Med Brain Sci 19(7):617–620
Lockshin RA, Zakeri Z (2004) Apoptosis, autophagy and more. Inter J Biochem Cell Biol 36(12):2405–2419
Lu YH, Yuan XD, Ou Y et al (2012) Autophagy and apoptosis during adult adipose-derived stromal cells differentiation into neuron-like cells in vitro. Neural Regen Res 7(16):1205–1212
Lu YH, Yuan XD, Ou Y, Cai YN, Sun QY (2013) In vitro amplification and ultrastructure of adult adipose-derived stromal cells. Chinese Journal of Tissue Engineering Research 17(10):1724–1729
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451(7182):1069–1075
Nakatsu Y, Kotake Y, Takai N, Ohta S (2010) Involvement of autophagy via mammalian target of rapamycin (mTOR) inhibition in tributyltin-induced neuronal cell death. J Toxicol Sci 35(2):245–251
Ou Y, Yuan XD, Cai YN, Lu YH (2011) A novel ethanol-based method to induce differentiation of adipose-derived stromal cells into astrocytes. Neural Regen Res 6(10):738–743
Reggiori F, Klionsky DJ (2002) Autophagy in the eukaryotic cell. Eukaryot Cell 1(1):11–21
Safford KM, Hicok KC, Safford SD et al (2002) Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun 294(2):371–379
Schmelzle T, Hall MN (2000) TOR, a central controller of cell growth. Cell 103(2):253–262
Tanida I, Ueno T, Kominami E (2004) LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol 36(12):2503–2518
Vicencio JM, Galluzzi L, Tajeddine N et al (2008) Senescence, apoptosis or autophagy? When a damaged cell must decide its path—a mini-review. Gerontology 54:92–99
Wei Y, Gong K, Zheng Z et al (2010) Schwann-like cell differentiation of rat adipose-derived stem cells by indirect co-culture with Schwann cells in vitro. Cell Prolif 43(6):606–616
Wrage PC, Tran T, To K et al (2008) The neuro-glial properties of adipose-derived adult stromal (ADAS) cells are not regulated by Notch 1 and are not derived from neural crest lineage. PLoS One 3(1):1453
Yang YP, Liang ZQ, Gu ZL, Qin ZH (2005) Molecular mechanism and regulation of autophagy. Acta Pharmacol Sin 26(12):1421–1434
Yang Q, She H, Gearing M et al (2009) Regulation of neuronal survival factor MEF2D by chaperone-mediated autophagy. Science 323(5910):124–127
Yang GM, Gao P, Zheng J (2010) Autophagy: executor and manager of programmed cell death. Medical Recapitulate 16(14):2106–2109
Ye CQ, Yuan XD, Liu H, Cai YN, Ou Y (2010) Ultrastructure of neuronal-like cells differentiated from adult adipose-derived stromal cells. Neural Regen Res 5(19):1456–1463
Zhang ZC, Shao ZW (2008) Research progress on autophagy molecule mechanism. Progress in Modem Biomedicine 8(1):177–179
Zustiak MP, Pollack JK, Marten MR, Betenbaugh MJ (2008) Feast or famine: autophagy control and engineering in eukaryotic cell culture. Curr Opin Biotechnol 19(5):518–526
Acknowledgments
The authors would like to thank director Yang Xiaolin and Ms. Yu Bo of Cosmetic Plastic Surgery Center from Kailuan General Hospital. This work gained equipment supports and technical assistance of teachers from the Experimental Center of Hebei United University.
Conflict of Interest
The study did not receive economic or interests sponsorship from any manufacturers, employers, or other economic organizations directly or indirectly.
Ethical Requirements
Volunteers who participated in the surgery had agreed to donate their adipose tissue for scientific research and signed “the informed consent”. The study received the approval of Medical Ethics Committee from Affiliated Kailuan General Hospital of Hebei United University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, Q., Ou, Y., Wang, S. et al. The Effect of Autophagy in the Process of Adipose-Derived Stromal Cells Differentiation into Astrocytes. J Mol Neurosci 53, 608–616 (2014). https://doi.org/10.1007/s12031-014-0227-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-014-0227-5