Abstract
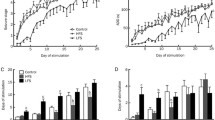
Deep brain stimulation is an alternate treatment strategy for intractable epilepsy. The effects of low- and high-frequency electrical stimulation to the substantia nigra pars reticulata (SNr) of different sides on chemically induced neocortical seizure were investigated in the present study. After neocortical seizure was induced by ferric chloride injection into the left sensorimotor cortex, SNr was stimulated ipsilaterally, contralaterally, or bilaterally at frequencies of 130 or 20 Hz in rats. Unilateral and bilateral stimulation at 130 Hz reduced significantly the number of seizures but not their duration. Ipsilateral, contralateral as well as bilateral stimulations at 130 Hz were all equally effective, producing reductions in seizures of 63.62, 77.84, and 68.74 % compared with the control group, respectively. Electrical stimulation at 20 Hz did not reduce the number or duration of seizures regardless of the side stimulated. Both unilateral and bilateral stimulations of SNr at 130 Hz can suppress ictogenesis in the cortex, but electrical stimulation at 130 or 20 Hz does not reduce the severity of individual seizures. The frequency of stimulation is paramount in suppressing neocortical seizures in which DBS at least targets SNr.




Similar content being viewed by others
References
Albert GC, Cook CM, Prato FS, Thomas AW (2009) Deep brain stimulation, vagal nerve stimulation and transcranial stimulation: an overview of stimulation parameters and neurotransmitter release. Neurosci Biobehav Rev 33:1042–1060
Benabid AL (2003) Deep brain stimulation for Parkinson’s Disease. Curr Opin Neurobiol 13:696–706
Beurrier C, Bioulac B, Audin J, Hammond C (2001) High-frequency stimulation produces a transient blockage of voltage-gated currents in subthalamic neurons. J Neurophysiol 85:1351–1356
Boda B, Szente MB (1992) Stimulation of substantia nigra pars reticulata suppresses neocortical seizures. Brain Res 574:237–243
Chabardes S, Kahane P, Minotti L, Koudsie A, Hirsch E, Benabid AL (2002) Deep brain stimulation in epilepsy with particular reference to the subthalamic nucleus. Epileptic Disord 4:S83–S93
Depaulis A, Vergnes M, Marescaux C (1994) Endogenous control of epilepsy: the nigral inhibitory system. Prog Neurobiol 42:33–52
Deransart C, Depaulis A (2002) The control of seizures by the basal ganglia? A review of experimental data. Epileptic Disord 4:S61–S72
Deransart C, Marescaux C, Depaulis A (1996) Involvement of nigral glutamatergic inputs in the control of seizures in a genetic model of absence epilepsy in the rat. Neurosci 71:721–728
Dostrovsky JO, Levy R, Wu JP, Hutchison WD, Tasker RR, Lozano AM (2000) Microstimulation-induced inhibition of neuronal firing in human globus pallidus. J Neurophysiol 84:570–574
Ellis TL, Stevens A (2008) Deep brain stimulation for medically refractory epilepsy. Neurosurg Focus 25:1–11
Feddersen B, Vercueil L, Noachtar S, David O, Depaulis A, Deransart C (2007) Controlling seizures is not controlling epilepsy: a parametric study of deep brain stimulation for epilepsy. Neurobiol Dis 27:292–300
Gale K (1992) Subcortical structures and pathways involved in convulsive seizure generation. J Clin Neurophysiol 9:264–277
Gerfen CR, Staines WA, Arbuthnott GW, Fibiger HC (1982) Crossed connections of the substantia nigra in the rat. J Comp Neurol 207:283–303
Goodman JH, Berger RE, Tcheng TK (2005) Preemptive low-frequency stimulation decreases the incidence of amygdala kindled seizures. Epilepsia 46:1–7
Gross RE (2008) Activation of subthalamic nucleus outflow by high-frequency stimulation is consistent with the nigral control of epilepsy model. Stereotact Funct Neurosurg 86:216–218
Hamani C, Ewerton FI, Bonilha SM, Ballester G, Mello LE, Lozano AM (2004) Bilateral anterior thalamic nucleus lesions and highfrequency stimulation are protective against pilocarpine-induced seizures and status epilepticus. Neurosurgery 54:191–197
Hsieh CL, Chang CH, Chiang SY, Li TC, Tang NY, Pon CZ, Hsieh CT, Lin JG (2000) Anticonvulsive and free radical scavenging activities of vanillyl alcohol in ferric chloride-induced epileptic seizures in Sprague–Dawley rats. Life Sci 67:1185–1195
Iadarola MJ, Gale K (1982) Substantia nigra: site of anticonvulsant activity mediated by g-aminobutyric acid. Science 218:1237–1240
Jiang H, Stein BE, McHaffie JG (2003) Opposing basal ganglia processes shape midbrain visuomotor activity bilaterally. Nature 423:982–986
Kahane P, Depaulis A (2010) Deep brain stimulation in epilepsy: what is next? Curr Opin Neurol 23:177–182
Lado FA, Velisek L, Moshe SL (2003) The effect of electrical stimulation of the subthalamic nucleus on seizures is frequency dependent. Epilepsia 44:157–164
Liu P, Basso MA (2008) Substantia nigra stimulation influences monkey superior colliculus neuronal activity bilaterally. J Neurophysiol 100:1098–1112
Loddenkemper T, Pan A, Neme S, Baker KB, Rezai AR, Dinner DS, Montgomery EB Jr, Lüders HO (2001) Deep brain stimulation in epilepsy. J Clin Neurophysiol 18:514–532
Merrill MA, Clough RW, Jobe PC, Browning RA (2003) Role of the superior colliculus and the intercollicular nucleus in the brainstem seizure circuitry of the genetically epilepsy-prone rat. Epilepsia 44:305–314
Merrill DR, Bikson M, Jefferys JG (2005) Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods 141:171–198
Meyerhoff JL, Lee JK, Rittase BW, Tsang AY, Yourick DL (2004) Lipoic acid pretreatment attenuates ferric chloride-induced seizures in the rat. Brain Res 6:139–144
Morimoto K, Goddard GV (1987) The substantia nigra is an important site for the containment of seizure generalization in the kindling model of epilepsy. Epilepsia 28:1–10
Morrell M (2006) Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Curr Opin Neurol 19:164–168
Paxinos G, Watson G (2005) The rat brain in stereotaxic coordinates, 5th edn. Elsevier, San Diego
Redgrave P, Marrow L, Dean P (1992a) Topographical organization of the nigrotectal projection in rat: evidence for segregated channels. Neuroscience 50:571–595
Redgrave P, Marrow LP, Dean P (1992b) Anticonvulsant role of nigrotectal projection in the maximal electroshock model of epilepsy. II. Pathways from substantia nigra pars lateralis and adjacent peripeduncular area to the dorsal midbrain. Neuroscience 46:391–406
Sabatino M, Gravante G, Ferraro G, Savatteri V, La Grutta V (1988) Inhibitory control by substantia nigra of generalized epilepsy in the cat. Epilepsy Res 2:380–386
Shi LH, Luo F, Woodward D, Chang JY (2006) Deep brain stimulation of the substantia nigra pars reticulata exerts long lasting suppression of amygdala-kindled seizures. Brain Res 1090:202–207
Shi LH, Luo F, Woodward DJ, McIntyre DC, Chang JY (2007) Temporal sequence of ictal discharges propagation in the corticolimbic basal ganglia system during amygdala kindled seizures in freely moving rats. Epilepsy Res 73:85–97
Sun HL, Zhang SH, Zhong K, Xu ZH, Zhu W, Fang Q, Wu DC, Hu WW, Xiao B, Chen Z (2010) Mode-dependent effect of low-frequency stimulation targeting the hippocampal CA3 subfield on amygdala-kindled seizures in rats. Epilepsy Res 90:83–90
Takebayashi S, Hashizume K, Tanaka T, Hodozuka A (2007) The effect of electrical stimulation and lesioning of the anterior thalamic nucleus on kainic acid-induced focal cortical seizure status in rats. Epilepsia 48:348–358
Usui N, Maesawa S, Kajita Y, Endo O, Takebayashi S, Yoshida J (2005) Suppression of secondary generalization of limbic seizures by stimulation of subthalamic nucleus in rats. J Neurosurg 102:1122–1129
Velısek L, Velıskova J, Moshe SL (2002) Electrical stimulation of substantia nigra pars reticulata is anticonvulsant in adult and young male rats. Exp Neurol 173:145–152
Vercueil L, Benazzouz A, Deransart C, Bressand K, Marescaux C, Depaulis A, Benabid AL (1998) High-frequency stimulation of the subthalamic nucleus suppresses absence seizures in the rat: comparison with neurotoxic lesions. Epilepsy Res 31:39–46
Willmore LJ, Sypert GW, Munson JV, Hurd RW (1978) Chronic focal epileptiform discharges induced by injection of iron into rat and cat cortex. Science 200:1501–1503
Wyckhuys T, Raedt R, Vonck K, Wadman W, Boon P (2010) Comparison of hippocampal deep brain stimulation with high (130 Hz) and low frequency (5 Hz) on afterdischarges in kindled rats. Epilepsy Res 88:239–246
Yamamoto N, Kabuto H, Matsumoto S, Ogawa N, Yokoi I (2002) alpha-tocopheryl-l-ascorbate-2-O-phosphate diester, a hydroxyl radical scavenger, prevents the occurrence of epileptic foci in a rat model of post-traumatic epilepsy. Pathophysiology 8:205–214
Yao QH, Zhang H, Wang HW, Jing XR, Guo H, Guo DG (2008) Low- and high frequency electric cortical stimulation suppress the ferric chloride-induced seizures in rats. Neurosci Lett 430:187–190
Zhong XL, Yu JT, Zhang Q, Wang ND, Tan L (2011) Deep brain stimulation for epilepsy in clinical practice and in animal models. Brain Res Bull 85:81–88
Zhu-Ge ZB, Zhu YY, Wu DC, Wang S, Liu LY, Hu WW, Chen Z (2007) Unilateral low frequency stimulation of central piriform cortex inhibits amygdaloid-kindled seizures in Sprague–Dawley rats. Neuroscience 146:901–906
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant no. 30772221).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Hua Zhang is co-first author.
Rights and permissions
About this article
Cite this article
Guo, H., Zhang, H., Kuang, Y. et al. Electrical Stimulation of the Substantia Nigra Pars Reticulata (SNr) Suppresses Chemically Induced Neocortical Seizures in Rats. J Mol Neurosci 53, 546–552 (2014). https://doi.org/10.1007/s12031-013-0220-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-013-0220-4