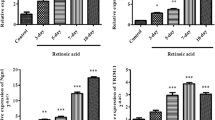
Abstract
Malignant peripheral nerve sheath tumors (MPNSTs) are sarcomas able to grow under conditions of metabolic stress caused by insufficient nutrients or oxygen. Both pituitary adenylate cyclase-activating polypeptide (PACAP) and activity-dependent neuroprotective protein (ADNP) have glioprotective potential. However, whether PACAP/ADNP signaling is involved in the resistance to cell death in MPNST cells remains to be clarified. Here, we investigated the involvement of this signaling system in the survival response of MPNST cells against hydrogen peroxide (H2O2)-evoked death both in the presence of normal serum (NS) and in serum-starved (SS) cells. Results showed that ADNP levels increased time-dependently (6–48 h) in SS cells. Treatment with PACAP38 (10−9 to 10−5 M) dose-dependently increased ADNP levels in NS but not in SS cells. PAC1/VPAC receptor antagonists completely suppressed PACAP-stimulated ADNP increase and partially reduced ADNP expression in SS cells. NS-cultured cells exposed to H2O2 showed significantly reduced cell viability (~50 %), increased p53 and caspase-3, and DNA fragmentation, without affecting ADNP expression. Serum starvation significantly reduced H2O2-induced detrimental effects in MPNST cells, which were not further ameliorated by PACAP38. Altogether, these finding provide evidence for the involvement of an endogenous PACAP-mediated ADNP signaling system that increases MPNST cell resistance to H2O2-induced death upon serum starvation.





Similar content being viewed by others
References
Ago Y, Yoneyama M, Ishihama T et al (2011) Role of endogenous pituitary adenylate cyclase-activating polypeptide in adult hippocampal neurogenesis. Neuroscience 172:554–561
Bassan M, Zamostiano R, Davidson A et al (1999) Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J Neurochem 72:1283–1293
Blouw B, Song H, Tihan T et al (2003) The hypoxic response of tumors is dependent on their microenvironment. Canc Cell 4:133–146
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carroll SL, Ratner N (2008) How does the Schwann cell lineage form tumors in NF1? Glia 56:1590–1605
Castorina A, Tiralongo A, Giunta S, Carnazza ML, Rasi G, D’Agata V (2008) PACAP and VIP prevent apoptosis in schwannoma cells. Brain Res 1241:29–35
Dejda A, Sokołowska P, Nowak JZ (2005) Neuroprotective potential of three neuropeptides PACAP, VIP and PHI. Pharmacol Rep 57:307–320
Forloni G, Angeretti N, Chiesa N et al (1993) Neurotoxicity of a prion protein fragment. Nature 362:543–546
Friedman JM, Gutmann DH, MacCollin M, Ricardi VM (1999) Neurofibromatosis: phenotype, natural history, and pathogenesis, 3rd edn. The Johns Hopkins University Press, Baltimore
Giladi E, Hill JM, Dresner E et al (2007) Vasoactive intestinal peptide (VIP) regulates activity-dependent neuroprotective protein (ADNP) expression in vivo. J Mol Neurosci 33:278–283
Gozes I, Divinsky I, Pilzer I et al (2003) From vasoactive intestinal peptide (VIP) through activity-dependent neuroprotective protein (ADNP) to NAP: a view of neuroprotection and cell division. J Mol Neurosci 20:315–322
Hamdi Y, Masmoudi-Kouki O, Kaddour H et al (2011) Protective effect of the octadecaneuropeptide on hydrogen peroxide-induced oxidative stress and cell death in cultured rat astrocytes. J Neurochem 118:416–428
Harmar AJ, Fahrenkrug J, Gozes I, et al. (2012) IUPHAR Reviews 1: Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Br J Pharmacol (in press)
Heasley LE (2001) Autocrine and paracrine signaling through neuropeptide receptors in human cancer. Oncogene 20:1563–1569
Hoelzinger DB, Demuth T, Berens ME (2007) Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst 99:1583–1593
Kenney-Herbert EM, Ball SL, Al-Mayhani TM, Watts C (2011) Glioblastoma cell lines derived under serum-free conditions can be used as an in vitro model system to evaluate therapeutic response. Cancer Lett 305:50–57
Laburthe M, Couvineau A (2002) Molecular pharmacology and structure of VPAC receptors for VIP and PACAP. Regul Pept 108:165–173
Lelievre V, Ghiani CA, Seksenyan A, Gressens P, de Vellis J, Waschek JA (2006) Growth factor-dependent actions of PACAP on oligodendrocyte progenitor proliferation. Regul Pept 137:58–66
Li M, David C, Kikuta T, Somogyvari-Vigh A, Arimura A (2005) Signaling cascades involved in neuroprotection by subpicomolar pituitary adenylate cyclase-activating polypeptide 38. J Mol Neurosci 27:91–105
Lutz EM, Ronaldson E, Shaw P, Johnson MS, Holland PJ, Mitchell R (2006) Characterization of novel splice variants of the PAC1 receptor in human neuroblastoma cells: consequences for signaling by VIP and PACAP. Mol Cell Neurosci 31:193–209
Masmoudi-Kouki O, Gandolfo P, Leprince J et al (2003) Pituitary adenylate cyclase-activating polypeptide (PACAP) stimulates endozepine release from cultured rat astrocytes via a PKA-dependent mechanism. FASEB J 17:17–27
Masmoudi-Kouki O, Douiri S, Hamdi Y et al (2011) Pituitary adenylate cyclase-activating polypeptide protects astroglial cells against oxidative stress-induced apoptosis. J Neurochem 117:403–411
Miyata A, Arimura A, Dahl RR et al (1989) Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 164:567–574
Nakamachi T, Li M, Shioda S, Arimura A (2006) Signaling involved in pituitary adenylate cyclase-activating polypeptide-stimulated ADNP expression. Peptides 27:1859–1864
Pascale A, Fortino I, Covoni S, Trabucchi M, Wetsel WC, Battaini F (1996) Functional impairment in protein kinase C by RACK1 (receptor for activated C kinase 1) deficiency in aged rat brain cortex. J Neurochem 67:2471–2477
Perrone F, Da Riva L, Orsenigo M et al (2009) PDGFRA, PDGFRB, EGFR, and downstream signaling activation in malignant peripheral nerve sheath tumor. Neuro Oncol 11:725–736
Pinhasov A, Mandel S, Torchinsky A et al (2003) Activity-dependent neuroprotective protein: a novel gene essential for brain formation. Brain Res Dev Brain Res 144:83–90
Ravni A, Bourgault S, Lebon A et al (2006) The neurotrophic effects of PACAP in PC12 cells: control by multiple transduction pathways. J Neurochem 98:321–329
Reglodi D, Kiss P, Lubics A, Tamas A (2011) Review on the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr Pharm Des 17:962–972
Rouzaire-Dubois B, Gérard V, Dubois JM (1993) Involvement of K+ channels in the quercetin-induced inhibition of neuroblastoma cell growth. Pflugers Arch 423:202–205
Sangueza OP, Requena L (1998) Neoplasms with neural differentiation: a review. Part II. Malignant neoplasms. Am J Dermapathol 20:89–102
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Sigalov E, Fridkin M, Brenneman DE, Gozes I (2000) VIP-related protection against lodoacetate toxicity in pheochromocytoma (PC12) cells: a model for ischemic/hypoxic injury. J Mol Neurosci 15:147–154
Steingart RA, Gozes I (2006) Recombinant activity-dependent neuroprotective protein protects cells against oxidative stress. Mol Cell Endocrinol 252:148–153
Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H (2000) Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev 52:269–324
Xie Y, Wolff DW, Lin MF, Tu Y (2007) Vasoactive intestinal peptide transactivates the androgen receptor through a protein kinase A-dependent extracellular signal-regulated kinase pathway in prostate cancer LNCaP cells. Mol Pharmacol 72:73–85
Zamostiano R, Pinhasov A, Gelber E et al (2001) Cloning and characterization of the human activity-dependent neuroprotective protein. J Biol Chem 276:708–714
Zhou CJ, Shioda S, Yada T, Inagaki N, Pleasure SJ, Kikuyama S (2002) PACAP and its receptors exert pleiotropic effects in the nervous system by activating multiple signaling pathways. Curr Protein Pept Sci 3:423–439
Zusev M, Gozes I (2004) Differential regulation of activity-dependent neuroprotective protein in rat astrocytes by VIP and PACAP. Regul Pept 123:33–41
Acknowledgments
These experiments were partially funded by Ministero dell’Istruzione, dell’Università e della Ricerca (PRIN grant no. 2007SXKWSA) and were supported by the International PhD program in Neuropharmacology, University of Catania, Medical School. We thank Mr P. Asero for his technical support and Dr. F. Murabito for his administrative support. All authors declare that there are no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castorina, A., Giunta, S., Scuderi, S. et al. Involvement of PACAP/ADNP Signaling in the Resistance to Cell Death in Malignant Peripheral Nerve Sheath Tumor (MPNST) Cells. J Mol Neurosci 48, 674–683 (2012). https://doi.org/10.1007/s12031-012-9755-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-012-9755-z