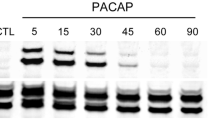
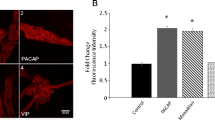
Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) increases neurite outgrowth, although signaling via its receptor PACAP-specific receptor (PAC1R) has not been fully characterized. Because mitochondria also play an important role in neurite outgrowth, we examined whether mitochondria contribute to PACAP-mediated neurite outgrowth. When mouse primary hippocampal neurons and Neuro2a cells were exposed to PACAP, neurite outgrowth and the mitochondrial membrane potential increased in both cell types. These results were reproduced using the PAC1R-specific agonist maxadilan and the adenylate cyclase activator forskolin, whereas the protein kinase A inhibitor H89 and mitochondrial uncoupling agent carbonyl cyanide m-chlorophenyl hydrazone (CCCP) inhibited these effects. Expression levels of peroxisome proliferator-activated receptor γ coactivator 1α (Pgc1α), a master regulator of mitochondrial activation, and its downstream effectors, such as cytochrome C and cytochrome C oxidase subunit 4, increased in response to PACAP. Knocking down Pgc1α expression using small interfering RNA or treatment with CCCP significantly attenuated neurite outgrowth and reduced the mitochondrial membrane potential in PACAP-treated cells. These data suggest that mitochondrial activation plays a key role in PACAP-induced neurite outgrowth via a signaling pathway that includes PAC1R, PKA, and Pgc1α.






Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- ATP:
-
Adenosine triphosphate
- CCCP:
-
Carbonyl cyanide m-chlorophenyl hydrazone
- COX4:
-
Cytochrome C oxidase subunit 4
- CREB:
-
cAMP response element binding protein
- CytC:
-
Cytochrome C
- DIV:
-
Days in vitro
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FBS:
-
Fetal bovine serum
- JC-1:
-
5,5′,6,6′-tetra-chloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide
- MAP2:
-
Microtubule-associated protein 2
- Δψ m :
-
Mitochondrial membrane potential
- PBS:
-
Phosphate-buffered saline
- PACAP:
-
Pituitary adenylate cyclase-activating polypeptide
- PAC1R:
-
PACAP-specific receptor
- Pgc1α:
-
Peroxisome proliferator-activated receptor γ coactivator 1α
- PKA:
-
Protein kinase A
- siRNA:
-
Small interfering RNA
References
Aglah C, Gordon T, Posse De Chaves EI (2008) cAMP promotes neurite outgrowth and extension through protein kinase A but independently of Erk activation in cultured rat motoneurons. Neuropharmacol 55:8–17
Cheng A, Hou Y, Mattson MP (2010) Mitochondria and neuroplasticity. ASN Neuro 2:e00045
Christensen AE, Selheim F, De Rooij J et al (2003) cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem 278:35394–35402
Dickey AS, Strack S (2011) PKA/AKAP1 and PP2A/Bbeta2 regulate neuronal morphogenesis via Drp1 phosphorylation and mitochondrial bioenergetics. J Neurosci 31:15716–15726
Estrada M, Uhlen P, Ehrlich BE (2006) Ca2+ oscillations induced by testosterone enhance neurite outgrowth. J Cell Sci 119:733–743
Falluel-Morel A, Aubert N, Vaudry D et al (2004) Opposite regulation of the mitochondrial apoptotic pathway by C2-ceramide and PACAP through a MAP-kinase-dependent mechanism in cerebellar granule cells. J Neurochem 91:1231–1243
Guirland C, Buck KB, Gibney JA, Dicicco-Bloom E, Zheng JQ (2003) Direct cAMP signaling through G-protein-coupled receptors mediates growth cone attraction induced by pituitary adenylate cyclase-activating polypeptide. J Neurosci 23:2274–2283
Hansel DE, Eipper BA, Ronnett GV (2001) Regulation of olfactory neurogenesis by amidated neuropeptides. J Neurosci Res 66:1–7
Horvath G, Racz B, Reglodi D et al (2010) Effects of PACAP on mitochondrial apoptotic pathways and cytokine expression in rats subjected to renal ischemia/reperfusion. J Mol Neurosci 42:411–418
Ishihara N, Nomura M, Jofuku A et al (2009) Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol 11:958–966
Kambe Y, Nakamichi N, Georgiev DD, Nakamura N, Taniura H, Yoneda Y (2008) Insensitivity to glutamate neurotoxicity mediated by NMDA receptors in association with delayed mitochondrial membrane potential disruption in cultured rat cortical neurons. J Neurochem 105:1886–1900
Kelly DP, Scarpulla RC (2004) Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev 18:357–368
Lai L, Leone TC, Zechner C, Schaeffer PJ et al (2008) Transcriptional coactivators PGC-1alpha and PGC-lbeta control overlapping programs required for perinatal maturation of the heart. Genes Dev 22:1948–1961
Lee CW, Peng HB (2008) The function of mitochondria in presynaptic development at the neuromuscular junction. Mol Biol Cell 19:150–158
Li Z, Okamoto K, Hayashi Y, Sheng M (2004) The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119:873–887
Lin J, Wu PH, Tarr PT et al (2004) Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 119:121–135
Mansouri S, Ortsater H, Pintor Gallego O, Darsalia V, Sjoholm A, Patrone C (2011) Pituitary adenylate cyclase-activating polypeptide counteracts the impaired adult neural stem cell viability induced by palmitate. J Neurosci Res 4:759–768
Maruoka H, Sasaya H, Shimamura Y, Nakatani Y, Shimoke K, Ikeuchi T (2010) Dibutyryl-cAMP up-regulates nur77 expression via histone modification during neurite outgrowth in PC12 cells. J Biochem 148:93–101
Mattson MP, Partin J (1999) Evidence for mitochondrial control of neuronal polarity. J Neurosci Res 56:8–20
Mcilvain HB, Baudy A, Sullivan K, Liu D, Pong K, Fennell M, Dunlop J (2006) Pituitary adenylate cyclase-activating peptide (PACAP) induces differentiation in the neuronal F11 cell line through a PKA-dependent pathway. Brain Res 1077:16–23
Miyata A, Arimura A, Dahl RR et al (1989) Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 164:567–574
Monaghan TK, Mackenzie CJ, Plevin R, Lutz EM (2008) PACAP-38 induces neuronal differentiation of human SH-SY5Y neuroblastoma cells via cAMP-mediated activation of ERK and p38 MAP kinases. J Neurochem 104:74–88
Racz B, Gallyas F Jr, Kiss P et al (2007) Effects of pituitary adenylate cyclase activating polypeptide (PACAP) on the PKA-Bad-14-3-3 signaling pathway in glutamate-induced retinal injury in neonatal rats. Neurotox Res 12:95–104
Sheward WJ, Lutz EM, Copp AJ, Harmar AJ (1998) Expression of PACAP, and PACAP type 1 (PAC1) receptor mRNA during development of the mouse embryo. Brain Res Dev Brain Res 109:245–253
Smiley ST, Reers M, Mottola-Hartshorn C et al (1991) Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci U S A 88:3671–3675
St-Pierre J, Drori S, Uldry M et al (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127:397–408
Vaudry D, Stork PJ, Lazarovici P, Eiden LE (2002) Signaling pathways for PC12 cell differentiation: making the right connections. Science 296:1648–1649
Vaudry D, Falluel-Morel A, Bourgault S et al (2009) Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61:283–357
Verburg J, Hollenbeck PJ (2008) Mitochondrial membrane potential in axons increases with local nerve growth factor or semaphorin signaling. J Neurosci 28:8306–8315
Waschek JA, Casillas RA, Nguyen TB, Dicicco-Bloom EM, Carpenter EM, Rodriguez WI (1998) Neural tube expression of pituitary adenylate cyclase-activating peptide (PACAP) and receptor: potential role in patterning and neurogenesis. Proc Natl Acad Sci U S A 95:9602–9607
Watanabe J, Nakamachi T, Matsuno R et al (2007) Localization, characterization and function of pituitary adenylate cyclase-activating polypeptide during brain development. Peptides 28:1713–1719
Zhou CJ, Shioda S, Shibanuma M et al (1999) Pituitary adenylate cyclase-activating polypeptide receptors during development: expression in the rat embryo at primitive streak stage. Neurosci 93:375–391
Acknowledgments
This work was supported by a Grant-in-Aid for Young Scientists (B) (22790256) from The Ministry of Education, Culture, Sports, Science and Technology (MEXT, Japan). We wish to thank Drs. Takashi Kurihara and Kazuhiko Inoue for the helpful discussions and the Joint Research Laboratory (Kagoshima University Graduate School of Medical and Dental Sciences) for the use of facilities and equipment.
Conflicts of Interest
The authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kambe, Y., Miyata, A. Role of Mitochondrial Activation in PACAP Dependent Neurite Outgrowth. J Mol Neurosci 48, 550–557 (2012). https://doi.org/10.1007/s12031-012-9754-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-012-9754-0