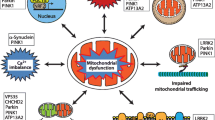
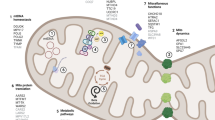
Abstract
Mitochondrial dysfunction has been implicated in the pathogenesis of sporadic, idiopathic Parkinson disease. In some cases, mitochondrial DNA primary genetic abnormalities, or more commonly, secondary rearrangements due to polymerase gamma (POLG1) gene mutation, can directly cause parkinsonism. The case of a Parkinson disease patient with some signs or symptoms suggestive of mitochondrial disease (i.e., ptosis, myopathy, neuropathy) is a relatively common event in the neurological practice. Mitochondrial parkinsonisms do not have distinctive features allowing an immediate diagnosis, and a negative family history does not rule out a possible diagnosis of mitochondrial disorder. In this article, we do not revise the mitochondrial hypothesis of sporadic, idiopathic Parkinson disease, extensively discussed elsewhere, but we review POLG1-related parkinsonism and other well-defined forms of “mitochondrial parkinsonisms”, with mtDNA mutations or rearrangements. Lastly, we try to introduce a possible diagnostic approach for patients with parkinsonism and suspected mitochondrial disorder.


Similar content being viewed by others
References
Anvret A, Westerlund M, Sydow O et al (2010) Variations of the CAG trinucleotide repeat in DNA polymerase gamma (POLG1) is associated with Parkinson’s disease in Sweden. Neurosci Lett 485:117–120
Autere J, Moilanen JS, Finnila S et al (2004) Mitochondrial DNA polymorphisms as risk factors for Parkinson’s disease and Parkinson’s disease dementia. Hum Genet 115:29–35
Baloh RH, Salavaggione E, Milbrandt J, Pestronk A (2007) Familial parkinsonism and ophthalmoplegia from a mutation in the mitochondrial DNA helicase twinkle. Arch Neurol 64:998–1000
Batabyal D, McKenzie JL, Johnson KA (2010) Role of histidine-932 of the human mitochondrial DNA polymerase in nucleotide discrimination and inherited disease. J Biol Chem 285:34191–34201
Betts-Henderson J, Jaros E, Krishnan KJ et al (2009) Alpha-synuclein pathology and Parkinsonism associated with POLG1 mutations and multiple mitochondrial DNA deletions. Neuropathol Appl Neurobiol 35:120–124
Blok MJ, van den Bosch BJ, Jongen E et al (2009) The unfolding clinical spectrum of POLG mutations. J Med Genet 46:776–785
Bueler H (2009) Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson’s disease. Exp Neurol 218:235–246
Chan SS, Copeland WC (2009) DNA polymerase gamma and mitochondrial disease: understanding the consequence of POLG mutations. Biochim Biophys Acta 1787:312–319
Chan SS, Longley MJ, Copeland WC (2005) The common A467T mutation in the human mitochondrial DNA polymerase (POLG) compromises catalytic efficiency and interaction with the accessory subunit. J Biol Chem 280:31341–31346
Copeland WC (2010) The mitochondrial DNA polymerase in health and disease. Subcell Biochem 50:211–222
Craig K., Ferrari G., Tiangyou W., et al. (2007) The A467T and W748S POLG substitutions are a rare cause of adult-onset ataxia in Europe. Brain 130, E69; author reply E70.
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909
Davidzon G, Greene P, Mancuso M et al (2006) Early-onset familial parkinsonism due to POLG mutations. Ann Neurol 59:859–862
De Coo IF, Renier WO, Ruitenbeek W et al (1999) A 4-base pair deletion in the mitochondrial cytochrome b gene associated with parkinsonism/MELAS overlap syndrome. Ann Neurol 45:130–133
DiMauro S, Schon EA (2003) Mitochondrial respiratory-chain diseases. N Engl J Med 348:2656–2668
Echaniz-Laguna A, Chassagne M, de Seze J et al (2010) POLG1 variations presenting as multiple sclerosis. Arch Neurol 67:1140–1143
Eerola J, Luoma PT, Peuralinna T et al (2010) POLG1 polyglutamine tract variants associated with Parkinson’s disease. Neurosci Lett 477:1–5
Filosto M, Mancuso M, Nishigaki Y et al (2003) Clinical and genetic heterogeneity in progressive external ophthalmoplegia due to mutations in polymerase gamma. Arch Neurol 60:1279–1284
Fukae J, Mizuno Y, Hattori N (2007) Mitochondrial dysfunction in Parkinson’s disease. Mitochondrion 7:58–62
Galassi G, Lamantea E, Invernizzi F et al (2008) Additive effects of POLG1 and ANT1 mutations in a complex encephalomyopathy. Neuromuscul Disord 18:465–470
Ghezzi D, Marelli C, Achilli A et al (2005) Mitochondrial DNA haplogroup K is associated with a lower risk of Parkinson’s disease in Italians. Eur J Hum Genet 13:748–752
Gonzalez-Vioque E, Blazquez A, Fernandez-Moreira D et al (2006) Association of novel POLG mutations and multiple mitochondrial DNA deletions with variable clinical phenotypes in a Spanish population. Arch Neurol 63:107–111
Graziewicz MA, Bienstock RJ, Copeland WC (2007) The DNA polymerase gamma Y955C disease variant associated with PEO and parkinsonism mediates the incorporation and translesion synthesis opposite 7, 8-dihydro-8-oxo-2′-deoxyguanosine. Hum Mol Genet 16:2729–2739
Gu M, Cooper JM, Taanman JW, Schapira AH (1998) Mitochondrial DNA transmission of the mitochondrial defect in Parkinson’s disease. Ann Neurol 44:177–186
Hakonen AH, Heiskanen S, Juvonen V et al (2005) Mitochondrial DNA polymerase W748S mutation: a common cause of autosomal recessive ataxia with ancient European origin. Am J Hum Genet 77:430–441
Harris MO, Walsh LE, Hattab EM, Golomb MR (2010) Is it ADEM, POLG, or both? Arch Neurol 67:493–496
Horvath R, Hudson G, Ferrari G et al (2006) Phenotypic spectrum associated with mutations of the mitochondrial polymerase gamma gene. Brain 129:1674–1684
Horvath R, Kley RA, Lochmuller H, Vorgerd M (2007) Parkinson syndrome, neuropathy, and myopathy caused by the mutation A8344G (MERRF) in tRNALys. Neurology 68:56–58
Hudson G. and Chinnery P.F. (2006) Mitochondrial DNA polymerase-gamma and human disease. Hum Mol Genet 15 Spec No 2, R244–252.
Hudson G, Schaefer AM, Taylor RW et al (2007) Mutation of the linker region of the polymerase gamma-1 (POLG1) gene associated with progressive external ophthalmoplegia and Parkinsonism. Arch Neurol 64:553–557
Huerta C, Castro MG, Coto E et al (2005) Mitochondrial DNA polymorphisms and risk of Parkinson’s disease in Spanish population. J Neurol Sci 236:49–54
Invernizzi F, Varanese S, Thomas A, Carrara F, Onofrj M, Zeviani M (2008) Two novel POLG1 mutations in a patient with progressive external ophthalmoplegia, levodopa-responsive pseudo-orthostatic tremor and parkinsonism. Neuromuscul Disord 18:460–464
Latsoudis H, Spanaki C, Chlouverakis G, Plaitakis A (2008) Mitochondrial DNA polymorphisms and haplogroups in Parkinson’s disease and control individuals with a similar genetic background. J Hum Genet 53:349–356
Luoma P, Melberg A, Rinne JO et al (2004) Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study. Lancet 364:875–882
Luoma PT, Luo N, Loscher WN et al (2005) Functional defects due to spacer-region mutations of human mitochondrial DNA polymerase in a family with an ataxia-myopathy syndrome. Hum Mol Genet 14:1907–1920
Luoma PT, Eerola J, Ahola S et al (2007) Mitochondrial DNA polymerase gamma variants in idiopathic sporadic Parkinson disease. Neurology 69:1152–1159
Mancuso M, Filosto M, Oh SJ, DiMauro S (2004) A novel polymerase gamma mutation in a family with ophthalmoplegia, neuropathy, and Parkinsonism. Arch Neurol 61:1777–1779
Mancuso M, Filosto M, Orsucci D, Siciliano G (2008a) Mitochondrial DNA sequence variation and neurodegeneration. Hum Genomics 3:71–78
Mancuso M, Nesti C, Petrozzi L et al (2008b) The mtDNA A8344G “MERRF” mutation is not a common cause of sporadic Parkinson disease in Italian population. Parkinsonism Relat Disord 14:381–382
Mancuso M, Orsucci D, Gori S, Ceravolo R, Siciliano G (2008c) Mitochondrial DNA single deletion in a patient with postural tremor. Mov Disord 23:2098–2100
Mancuso M, Orsucci D, Coppede F, Nesti C, Choub A, Siciliano G (2009) Diagnostic approach to mitochondrial disorders: the need for a reliable biomarker. Curr Mol Med 9:1095–1107
Milone M, Massie R (2010) Polymerase gamma 1 mutations: clinical correlations. Neurologist 16:84–91
Nikoskelainen EK, Marttila RJ, Huoponen K et al (1995) Leber’s “plus”: neurological abnormalities in patients with Leber’s hereditary optic neuropathy. J Neurol Neurosurg Psychiatry 59:160–164
Nishioka K, Vilarino-Guell C, Cobb SA et al (2010) Genetic variation of the mitochondrial complex I subunit NDUFV2 and Parkinson disease. Parkinsonism Relat Disord 16:686–687
Orsucci D, Filosto M, Siciliano G, Mancuso M (2009) Electron transfer mediators and other metabolites and cofactors in the treatment of mitochondrial dysfunction. Nutr Rev 67:427–438
Pagnamenta AT, Taanman JW, Wilson CJ et al (2006) Dominant inheritance of premature ovarian failure associated with mutant mitochondrial DNA polymerase gamma. Hum Reprod 21:2467–2473
Parker WD Jr, Boyson SJ, Parks JK (1989) Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann Neurol 26:719–723
Pyle A, Foltynie T, Tiangyou W et al (2005) Mitochondrial DNA haplogroup cluster UKJT reduces the risk of PD. Ann Neurol 57:564–567
Remes AM, Hinttala R, Karppa M et al (2008) Parkinsonism associated with the homozygous W748S mutation in the POLG1 gene. Parkinsonism Relat Disord 14:652–654
Sarzi E, Brown MD, Lebon S et al (2007) A novel recurrent mitochondrial DNA mutation in ND3 gene is associated with isolated complex I deficiency causing Leigh syndrome and dystonia. Am J Med Genet A 143:33–41
Schaefer AM, McFarland R, Blakely EL et al (2008) Prevalence of mitochondrial DNA disease in adults. Ann Neurol 63:35–39
Schicks Md J, Synofzik Md M, Schulte C, Schols Md L (2010) POLG, but not PEO1, is a frequent cause of cerebellar ataxia in Central Europe. Mov Disord 25:2678–2682
Siciliano G, Mancuso M, Ceravolo R, Lombardi V, Iudice A, Bonuccelli U (2001) Mitochondrial DNA rearrangements in young onset parkinsonism: two case reports. J Neurol Neurosurg Psychiatry 71:685–687
Simon DK, Friedman J, Breakefield XO et al (2003) A heteroplasmic mitochondrial complex I gene mutation in adult-onset dystonia. Neurogenetics 4:199–205
Simon DK, Pulst SM, Sutton JP, Browne SE, Beal MF, Johns DR (1999) Familial multisystem degeneration with parkinsonism associated with the 11778 mitochondrial DNA mutation. Neurology 53:1787–1793
Simon DK, Pankratz N, Kissell DK et al (2010) Maternal inheritance and mitochondrial DNA variants in familial Parkinson’s disease. BMC Med Genet 11:53
Solano A, Roig M, Vives-Bauza C et al (2003) Bilateral striatal necrosis associated with a novel mutation in the mitochondrial ND6 gene. Ann Neurol 54:527–530
Stewart JD, Hudson G, Yu-Wai-Man P et al (2008) OPA1 in multiple mitochondrial DNA deletion disorders. Neurology 71:1829–1831
Synofzik M, Asmus F, Reimold M, Schols L, Berg D (2010) Sustained dopaminergic response of parkinsonism and depression in POLG-associated parkinsonism. Mov Disord 25:243–245
Thyagarajan D, Bressman S, Bruno C et al (2000) A novel mitochondrial 12SrRNA point mutation in parkinsonism, deafness, and neuropathy. Ann Neurol 48:730–736
Trifunovic A, Wredenberg A, Falkenberg M et al (2004) Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429:417–423
Tzoulis C, Engelsen BA, Telstad W et al (2006) The spectrum of clinical disease caused by the A467T and W748S POLG mutations: a study of 26 cases. Brain 129:1685–1692
van der Walt JM, Nicodemus KK, Martin ER et al (2003) Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. Am J Hum Genet 72:804–811
Wang K, Takahashi Y, Gao ZL et al (2009) Mitochondrial ND3 as the novel causative gene for Leber hereditary optic neuropathy and dystonia. Neurogenetics 10:337–345
Wilcox RA, Churchyard A, Dahl HH, Hutchison WM, Kirby DM, Thyagarajan D (2007) Levodopa response in Parkinsonism with multiple mitochondrial DNA deletions. Mov Disord 22:1020–1023
Winterthun S, Ferrari G, He L et al (2005) Autosomal recessive mitochondrial ataxic syndrome due to mitochondrial polymerase gamma mutations. Neurology 64:1204–1208
Wong LJ, Naviaux RK, Brunetti-Pierri N et al (2008) Molecular and clinical genetics of mitochondrial diseases due to POLG mutations. Hum Mutat 29:E150–E172
Acknowledgment
This study was partially supported by Telethon Grant GUP09004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orsucci, D., Caldarazzo Ienco, E., Mancuso, M. et al. POLG1-Related and other “Mitochondrial Parkinsonisms”: an Overview. J Mol Neurosci 44, 17–24 (2011). https://doi.org/10.1007/s12031-010-9488-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-010-9488-9