Abstract
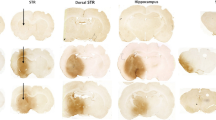
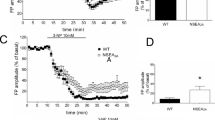
Somatostatin (SST) is a multifunctional peptide and involves in several neurodegenerative diseases. N-Methyl-d-asparate (NMDA) receptor agonist quinolinic acid (QUIN)-induced neurotoxicity mimics an experimental model of Huntington’s disease that is characterized by the selective preservation of medium-sized aspiny interneurons and degeneration of medium-sized spiny projection neurons in striatum. In QUIN- and NMDA-induced neurotoxicity, increased expression of SST and messenger RNA levels along with SST release in culture medium is generally observed. However, the molecular mechanisms and the functional consequences of increased SST are still obscure. In the present study, the role of SST was determined using immunoneutralization and immunoblockade of SST in cultured striatal neurons upon QUIN- and NMDA-induced neurotoxicity. The immunoblockade of SST with antisense oligonucleotides and immunoabsorption of released SST with specific antibodies potentiate QUIN- and NMDA-induced neuronal cell death. NADPH–diaphorase positive neurons that are selectively spared in several processes of neurodegeneration result in severe damage upon immunoblockade or immunoabsorption of SST. In addition, exogenous SST along with QUIN and NMDA provides selective preservation of projection neurons, which are selectively susceptible in excitotoxicity. Neuroprotective effect of SST is completely blocked by pertussis toxins, suggesting the role of somatostatin receptors. Taken together, these results provide first evidence that the presence of SST is a unique feature for the selective sparing of medium sized aspiny interneurons in excitotoxicity.






Similar content being viewed by others
References
Aarts, M., Liu, Y., Liu, L., Besshoh, S., Arudine, M., Gurd, J. W., et al. (2002). Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science, 298, 846–850.
Aronin, N., Coper, P. E., & Lorenz, L. J. (1983). Somatostatin is increased in the basal ganglia in Huntington’s disease. Annals of Neurology, 13, 519–526.
Augood, S. J., McGowan, E. K., & Emson, P. C. (1994). Expression of N-methyl-d-aspartate receptor subunit NR1 messenger RNA by identified striatal somatostatin cells. Neuroscience, 59, 7–12.
Beal, M. F., Ferrante, R. J., Swartz, K. J., & Kowall, N. W. (1991). Chronic quinolinic acid lesions in rats closely resemble Huntington’s disease. Journal of Neuroscience, 11, 1649–1659.
Beal, M. F., Kowall, N. W., Ellison, D. W., Mozurek, M. F., Swartz, K. J., & Martin, J. B. (1986). Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature, 321, 168–171.
Beal, M. F., Kowall, N. W., Swartz, K. J., Ferrante, R. J., & Martin, J. B. (1989). Differential sparing of somatostatin–neuropeptide Y and cholinergic neurons following striatal excitotoxin lesions. Synapse, 3(1), 38–47.
Beal, M. F., Masurek, M. F., Tran, V. T., Chattha, G., Bird, E. D., & Martin, M. B. (1985). Reduced numbers of somatostatin receptors in the cerebral cortex in Alzheimer’s disease. Science, 229, 289–291.
Bissette, G., Cook, L., Smith, W., Dole, K. C., Crain, B., & Nemeroff, C. B. (1998). Regional neuropeptide pathology in Alzheimer’s disease: Corticotropin-releasing factor and somatostatin. Journal of Alzheimer’s Disease, 1, 91–105.
Bissette, G., & Myers, B. (1992). Minireview: Somatostatin in Alzheimer’s disease and depression. Life Science, 51, 1389–1410.
Boegman, R. J., & Parent, A. (1988). Differential sensitivity of neuropeptide Y, somatostatin and NADPH-d containing neurons in rat cortex and striatum to quinolinic acid. Brain Research, 445, 358–362.
Boegman, R. J., Smith, Y., & Parent, A. (1987). Quinolinic acid does not spare striatal neuropeptide Y immunoreactive neurons. Brain Research, 415, 178–183.
Chen, Q., & Reiner, A. (1996). Cellular distribution of the NMDA receptor NR2A/2B subunits in rat striatum. Brain Research, 743, 346–352.
Davies, P., Katzman, R., & Terry, R. D. (1980). Reduced somatostatin-like immunoreactivity in cerebral cortex from cases of Alzheimer’s disease and Alzheimer senile dementia. Nature, 288, 279–280.
Davies, S. W., & Robert, P. J. (1987). No evidence for preservation of somatostatin-containing neurons after intrastriatal injections of quinolinic acid. Nature, 327, 326–329.
Davis, K. L., Davidson, M., Yang, R. K., Davis, B. M., Siever, L. J., Mohs, R. C., et al. (1988). CSF somatostatin in Alzheimer’s disease, depressed patients, and control subjects. Biological Psychiatry, 24, 710–712.
Dawbarn, D., De Quidt, M. E., & Emson, P. C. (1985). Survival of basal ganglia neuropeptide Y- somatostatin neurons in Huntington’s disease. Brain Research, 340, 251–260.
Dawson, V. L., Kizushi, V. M., Huang, P. L., Snyder, S. H., & Dawson, T. M. (1996). Resistance to neurotoxicity in cortical cultures from neuronal nitric oxide synthase deficient mice. Journal of Neuroscience, 16, 2479–2487.
Ferrante, R. J., Kowall, N. W., Beal, M. F., Richardson Jr., E. P., Bird, E. D., & Martin, J. B. (1985). Selective sparing of a class of striatal neurons in Huntington’s disease. Science, 230, 561–563.
Figueredo Cardenas, G., Chen, Q., & Reiner, A. (1997). Age-dependent differences in survival of striatal somatostatin–NPY–NADPH–diaphorase containing interneurons versus striatal projection neurons after intra-striatal injection of quinolinic acid in rat. Exper Neurology, 146, 444–457.
Forloni, G., Lucca, E., Angeretti, N., Chiesa, R., & Vezzani, A. (1997). Neuroprotective effect of somatostatin on non-apoptotic NMDA-induced neuronal death: role of cyclic GMP. Journal of Neurochemistry, 68(1), 319–327.
Geci, C., How, J., Alturahi, H., & Kumar, U. (2007). β-Amyloid increases the expression of Somatostatin mRNA its accumulation in cortical cultured neurons. Journal of Neurochemistry, 101, 664–673.
Hartley, D. M., Kurth, M. C., Bjerkness, L., Weiss, J. H., & Choi, D. W. (1993). Glutamate receptor induced 45Ca2+ accumulation in cortical cell culture correlates with subsequent neuronal degeneration. Journal of Neuroscience, 13, 1993–2000.
Hope, B. T., & Vincent, S. R. (1989). Histochemical characterization of neuronal NADPH-diaphorase. Journal of Histochemistry and Cytochemistry, 37, 653–661.
Huang, P. L., Dawson, T. M., Bredt, D. S., Snyder, S. H., & Fishman, M. C. (1993). Targeted disruption of neuronal nitric oxide synthase gene. Cell, 75, 1273–1286.
Koh, J. Y., & Choi, D. W. (1988). Cortical striatal neurons containing NADPH-d or acetylcholinetransferase are selectively resistant to injury by NMDA receptor agonist. Brain Research, 446, 374–378.
Koh, J. -Y., Peters, S., & Choi, D. W. (1986). Neurons containing NADPH-diaphorase are selectively resistant to quinolinate toxicity. Science, 234, 73–76.
Kumar, U. (2004). Characterization of striatal cultures with the effect of QUIN and NMDA. Neuroscience Research, 49, 29–38.
Kumar, U. (2005). Expression of somatostatin receptor subtypes (SSTR1–5) in Alzheimer’s disease brain: An immunohistochemical analysis. Neuroscience, 134, 525–538.
Kumar, U. (2007). Colocalization of somatostatin receptor subtypes (SSTR1–5) with somatostatin, tyrosine hydroxylase and NADPH-diaphorase in rat hypothalamus. Journal of Comparative Neurology, 504, 185–205.
Kumar, U., Asotra, K., Patel, S. C., & Patel, Y. C. (1997). Expression of NMDA receptor-1 (NR1) and huntingtin in striatal neurons which colocalize somatostatin, neuropeptide Y and NADPH-diaphorase: A double-label histochemical and immunohistochemical study. Experimental Neurology, 145, 412–424.
Martin, J. B., & Gusella, J. F. (1986). Huntington’s disease: Pathogenesis and management. New England Journal of Medicine, 315, 1267–1276.
Molchan, S. E., Hill, J. L., Martinez, R. A., Lawlor, B. A., Mellow, A. M., Rubino, D. R., et al. (1993). CSF somatostatin in Alzheimer’s disease, and major depression: Relationship to hypothalamic–pituitary–adrenal axis and clinical measures. Psychoneuroendrocrinol, 18, 509–519.
Monno, A., Rizzi, M., Samanin, R., & Vezzani, A. (1993). Anti-somatostatin antibody enhances the rate of hippocampal kindling. Brain Research, 602, 148–152.
Monyer, H., Sprengel, R., & Schoepjer, R. (1992). Heteromeric NMDA receptors: Molecular and functional distinction of subtypes. Science, 256, 1217–1221.
Nemeroff, C. B., Knight, D. L., & Bissette, G. (1992). Somatostatin: A neuropeptide system pathologically altered in Alzheimer’s disease and depression. Clinical Neuropharmacology, 15(Suppl), 311A–312A.
Nemeroff, C. B., Youngblood, W. W., Prange Jr., A. J., & Kizer, J. S. (1983). Regional brain concentrations of neuropeptides in Huntington’s chorea and schizophrenia. Science, 221, 972–975.
Nilsson, C. L., Brinkmalm, A., Minthon, L., Blenno, K., & Ekman, R. (2001). Processing of neuropeptide Y, galanin, and somatostatin in the cerebrospinal fluid of patients with Alzheimer’s disease and frontotemporal dementia. Peptides, 22, 2105–2112.
Norris, P. J., Waldvogel, H. J., Paul, R. L. M., Love, D. R., & Emson, P. C. (1996). Decreased neuronal nitric oxide synthase messenger RNA and somatostatin mRNA in the striatum of Huntington’s Disease. Neuroscience, 72, 1037–1047.
Patel, Y. C., Liu, J. L., Warszynska, A., Kent, G., Papachristou, D. N., & Patel, S. C. (1995). Differential stimulation of somatostatin but not neuropeptide Y gene expression by quinolinic acid in cultured cortical neurons. Journal of Neurochemistry, 65, 998–1006.
Patel, S. C., Papachristou, D. N., & Patel, Y. C. (1991). Quinolinic acid stimulates somatostatin gene expression in cultured rat cortical neurons. Journal of Neurochemistry, 56, 1286–1291.
Reiner, A., Albin, R. L., & Anderson, K. D. (1988). Differential loss of striatal projection neurons in Huntington’s disease. Proceedings of the National Academy of Sciences of the United States of America, 85, 5733–5737.
Saito, T., Iwata, N., Tsubuki, S., Takaki, Y., Takano, J., Huang, S. M., et al. (2005). Somatostatin regulates brain amyloid B peptide AB42 through modulation of proteolytic degradation. Nature Medicine, 11, 434–439.
Vezzani, A., Serafini, R., Stassi, M. A., Vigano, G., Rizzi, M., & Samanin, R. (1991). A peptidase-resistant cyclic octapeptide analogue of somatostatin (SMS 201-995) modulates seizure induced by quinolinic acid and kainic acids differently in the rat hippocampus. Neuropharmacology, 30, 345–352.
Vincent, S. R., & Johansson, O. (1983). Striatal neurons containing both somatostatin- and avian pancreatic polypeptide (APP)-like immunoreactivities and NADPH-diaphorase activity: A light and electron microscopic study. Journal of Comparative Neurology, 217, 264–270.
Wang, H. L., Reisine, T., & Dichter, M. (1990). Somatostatin-14 and somatostatin-28 inhibit calcium current in rat neocortical neurons. Neuroscience, 38, 335–342.
Williams, J. S., Berbekar, I., & Weiss, S. (1991). N-Methyl-d-aspartate evokes the release of somtostatin from striatal interneurons in primary culture. Neuroscience, 43, 437–444.
Young, A. B., Greenamyre, J. T., Hollingsworth, Z., Albin, R., D’Amato, C., Shoulson, I., et al. (1988). NMDA receptor losses in putamen from patients with Huntington’s disease. Science, 241, 981–983.
Zeron, M. M., Hansson, O., Chen, N., Wellington, C. L., Leavitt, B. R., Brudin, P., et al. (2002). Increased sensitivity to N-methyl-d-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington’s disease. Neuron, 33, 849–860.
Acknowledgment
This work was supported by grants from the Canadian Institute for Health Research (MOP-6196 and MOP-74465). The author thanks Haydar Alturaihi and Heather L. Watt for technical assistance, and Rania Mouchantaf and Aruna Rani for initial RIA for SST. The author is a Senior Investigator of the Michael Smith Foundation for Health Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, U. Somatostatin in Medium-Sized Aspiny Interneurons of Striatum is Responsible for Their Preservation in Quinolinic Acid and N-Methyl-d-Asparate-Induced Neurotoxicity. J Mol Neurosci 35, 345–354 (2008). https://doi.org/10.1007/s12031-008-9093-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-008-9093-3